Photosynthetic reaction centre
[1] A reaction center is laid out in such a way that it captures the energy of a photon using pigment molecules and turns it into a usable form.
In green plants, the electron transport chain has many electron acceptors including pheophytin, quinone, plastoquinone, cytochrome bf, and ferredoxin, which result finally in the reduced molecule NADPH, while the energy used to split water results in the release of oxygen.
Type I, found in green-sulfur bacteria, Heliobacteria, and plant/cyanobacterial PS-I, use iron sulfur clusters as electron acceptors.
[2][3] Cyanobacteria, the precursor to chloroplasts found in green plants, have both photosystems with both types of reaction centers.
[3] The bacterial photosynthetic reaction center has been an important model to understand the structure and chemistry of the biological process of capturing light energy.
However, the first crystal structure (upper image at right) was determined in 1984 by Hartmut Michel, Johann Deisenhofer and Robert Huber[4] for which they shared the Nobel Prize in 1988.
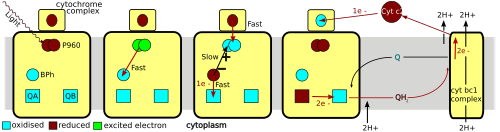
The L and M subunits, shown in blue and purple in the image of the structure, both span the lipid bilayer of the plasma membrane.
A cytochrome subunit, not shown here, contains four c-type hemes and is located on the periplasmic surface (outer) of the membrane.
Reaction centers from different bacterial species may contain slightly altered bacterio-chlorophyll and bacterio-pheophytin chromophores as functional co-factors.
The reaction center contains two pigments that serve to collect and transfer the energy from photon absorption: BChl and Bph.
The process starts when light is absorbed by two BChl molecules that lie near the periplasmic side of the membrane.
Cyanobacteria, the precursor to chloroplasts found in green plants, have both photosystems with both types of reaction centers.
In 1772, the chemist Joseph Priestley carried out a series of experiments relating to the gases involved in respiration and combustion.
In 1932, Robert Emerson and his student, William Arnold, used a repetitive flash technique to precisely measure small quantities of oxygen evolved by chlorophyll in the algae Chlorella.
Gaffron and Wohl later interpreted the experiment and realized that the light absorbed by the photosynthetic unit was transferred.
Photosystem II is present on the thylakoid membranes inside chloroplasts, the site of photosynthesis in green plants.
[9] The structure of Photosystem II is remarkably similar to the bacterial reaction center, and it is theorized that they share a common ancestor.
The fact that the oxygen from green plants originated from water was first deduced by the Canadian-born American biochemist Martin David Kamen.
Due to the presence of chlorophyll a, as opposed to bacteriochlorophyll, Photosystem II absorbs light at a shorter wavelength.
The difference between Photosystem II and the bacterial reaction center is the source of the electron that neutralizes the pair of chlorophyll a molecules.
In the bacterial reaction center, the electron is obtained from a reduced compound haem group in a cytochrome subunit or from a water-soluble cytochrome-c protein.
It passes its energy to water molecules that are bound at the manganese center directly below the pair and extracts an electron from them.
As with Photosystem II and the bacterial reaction center, a pair of chlorophyll a molecules initiates photoinduced charge separation.
The positive charge on the high-energy P700+ is neutralized by the transfer of an electron from plastocyanin, which receives energy eventually used to convert QH2 back to Q.