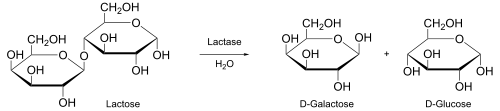
Product (chemistry)
[1] During a chemical reaction, reactants are transformed into products after passing through a high energy transition state.
When represented in chemical equations, products are by convention drawn on the right-hand side, even in the case of reversible reactions.
Ever since the mid-nineteenth century, chemists have been increasingly preoccupied with synthesizing chemical products.
[7] Disciplines focused on isolation and characterization of products, such as natural products chemists, remain important to the field, and the combination of their contributions alongside synthetic chemists has resulted in much of the framework through which chemistry is understood today.
Beginning in the early 2000s, process chemistry began emerging as a distinct field of synthetic chemistry focused on scaling up chemical synthesis to industrial levels, as well as finding ways to make these processes more efficient, safer, and environmentally responsible.
Some enzymes display a form of promiscuity where they convert a single substrate into multiple different products.