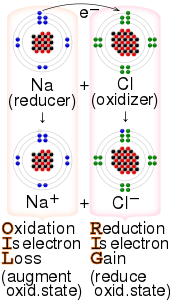
Reducing agent
For example, consider the overall reaction for aerobic cellular respiration: The oxygen (O2) is being reduced, so it is the oxidizing agent.
Good reducing agents tend to consist of atoms with a low electronegativity, which is the ability of an atom or molecule to attract bonding electrons, and species with relatively small ionization energies serve as good reducing agents too.
[citation needed] Common reducing agents include metals potassium, calcium, barium, sodium and magnesium, and also compounds that contain the hydride H− ion, those being NaH, LiH,[5] LiAlH4 and CaH2.
Hydrogen (whose reduction potential is 0.0) acts as an oxidizing agent because it accepts an electron donation from the reducing agent lithium (whose reduction potential is -3.04), which causes Li to be oxidized and hydrogen to be reduced.
The anode is an element that loses electrons (reducing agent), thus oxidation always occurs in the anode, and the cathode is an element that gains electrons (oxidizing agent), thus reduction always occurs in the cathode.
When this is present, the anode metal begins deteriorating, given there is an electrical connection and the presence of an electrolyte.
[7] An example of this phenomenon occurred during the Great Oxidation Event, in which biologically−produced molecular oxygen (dioxygen (O2), an oxidizer and electron recipient) was added to the early Earth's atmosphere, which was originally a weakly reducing atmosphere containing reducing gases like methane (CH4) and carbon monoxide (CO) (along with other electron donors)[8] and practically no oxygen because any that was produced would react with these or other reducers (particularly with iron dissolved in sea water), resulting in their removal.
By using water as a reducing agent, aquatic photosynthesizing cyanobacteria produced this molecular oxygen as a waste product.
[10] The modern sense of donating electrons is a generalization of this idea, acknowledging that other components can play a similar chemical role to oxygen.