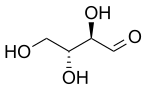
Erythrose
Erythrose is a tetrose saccharide with the chemical formula C4H8O4.
The natural isomer is D-erythrose; it is a diastereomer of D-threose.
[3] Erythrose was first isolated in 1849 from rhubarb by the French pharmacist Louis Feux Joseph Garot (1798-1869),[4] and was named as such because of its red hue in the presence of alkali metals (ἐρυθρός, "red").
[5][6] Erythrose 4-phosphate is an intermediate in the pentose phosphate pathway[7] and the Calvin cycle.
Oxidative bacteria can be made to use erythrose as its sole energy source.