Eclipsed conformation
This maximum is often explained by steric hindrance, but its origins sometimes actually lie in hyperconjugation (as when the eclipsing interaction is of two hydrogen atoms).
With this image in mind, if the methyl groups are rotated around the bond, they will remain connected; however, the shape will change.
Conformations can be described by dihedral angles, which are used to determine the placements of atoms and their distance from one another and can be visualized by Newman projections.
In the example of ethane, such a graph shows that rotation around the carbon-carbon bond is not entirely free but that an energy barrier exists.
Below is the sawhorse and Newman representation of butane in an eclipsed conformation with the two CH3 groups (C1 and C4) at a 0-degree angle from one another (left).
[5] Experiments such as X-ray and electron diffraction analyses, nuclear magnetic resonance, microwave spectroscopies, and more have allowed researchers to determine which cycloalkane structures are the most stable based on the different possible conformations.
In another case, 2,2,3,3-tetramethylbutane is shaped more like an ellipsoid causing it to be able to form a crystal lattice which raises the melting point of the molecule because it will take more energy to transition from a solid to a liquid state.
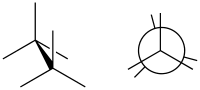
(image right in Newman projection )