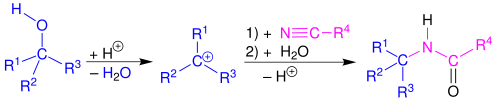Ritter reaction
The original reaction formed the alkylating agent using an alkene in the presence of a strong acid.
[1][2][3][4] The Ritter reaction proceeds by the electrophilic addition of either a carbenium ion or covalent species[5][6] to the nitrile.
Primary,[7] secondary,[4] tertiary,[8] and benzylic[9] alcohols,[1] as well as tert-butyl acetate,[10] also successfully react with nitriles in the presence of strong acids to form amides via the Ritter reaction.
[15] Other applications of the Ritter reaction include synthesis of dopamine receptor ligands[14] and production of racemic amphetamine from allylbenzene and methyl cyanide.
Other methods have been proposed in order to promote carbocation formation, including photocatalytic electron transfer[17] or direct photolysis.