Rosenthal's reagent
[5] Rosenthal's reagent can be prepared by reduction of titanocene or zirconocene dichloride with magnesium in the presence of bis(trimethylsilyl)acetylene in THF.
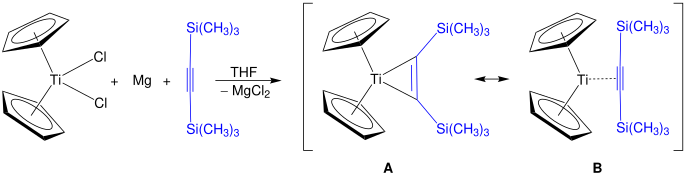
[6] The first successful synthesis of titanocene bis(trimethylsilyl)acetylene was accomplished by Uwe Rosenthal in 1988, via the reduction of Cp2TiCl2 with magnesium and the alkyne Me3SiC2SiMe3, in THF.
[1] The original synthesis involved the reduction of zirconocene dichloride and the addition of bis(trimethylsilyl)acetylene in THF before transferring to pyridine as mentioned above.
More recently, Tilley and coworkers demonstrated a simpler synthesis with a higher yield bypassing the isolation of the less stable THF adduct.
This newer method reacts zirconocene dichloride with 2 equivalents of n-Butyllithium in THF to form a metallacyclopropane which is subsequently substituted by bis(trimethylsilyl)acetylene and pyridine.
The two main resonance structures of Zirconocene bis(trimethylsilyl)acetylene pyridine include a variation where the C-C triple bond binds side on to the metal and another with the 1-metallacyclopropene configuration.
[16] However, a subsequent series of calculations by Leites and colleagues using a higher level of theory showed molecular orbitals more consistent with the triple bond description.
[17] The original creation of zirconocene bis(trimethylsilyl)acetylene pyridine was accompanied by reactivity studies of the complex with common small molecules in the form of carbon dioxide and water.
Both reactions involved the loss of the pyridine ligand and creation of bimetallic complexes containing bridging-oxo substituents, with the carbon dioxide inserting to create a series of fused metallacycles and the water’s hydrogen atoms breaking up the metallacyclopropenes.
[1] More generally, the main reactivity for this version of Rosenthal’s reagent is its reaction with alkynes to replace the zirconacyclopropene with a larger zirconacyclopentadiene rings.
[19] These large macrocycles can subsequently be reacted with hydrochloric acid to lose the zirconocene dichloride leaving behind new carbon-carbon bonds.
[20] Following these macrocycles, the Tilley group also showed that the zirconocene bis(trimethylsilyl)acetylene pyridine could aid in the creation of various polycyclic aromatic hydrocarbons via [2+2+n] cycloaddition reactions.
[23] Rosenthal also continued exploring the reactivity of the zirconocene bis(trimethylsilyl)acetylene pyridine showing the ability to functionalize the zirconacyclopentadienes in addition to modifying the ring itself.
These zirconium metallacycles can then be transmetalated to create functionalized stannoles which Staubitz later used in Stille cross coupling reactions to form polymers with thiophene groups.
In 2019, Ye and coworkers further extended the scope of the pyridine Rosenthal reagent reactivity, demonstrating its reaction with bis(alkylnyl)boranes in an attempt to create compounds capable of activating small molecules.
This was achieved by the reaction of Rosenthal’s reagent with Zr(Cp)2(CH3)(CCSiMe3) to create a methyl bridged complex which could be converted to the hydride upon the addition of BH3•NHMe2.
[31] Tonks and colleagues looked into the reactivity of this Rosenthal reagent as a potential ring opening complex, but instead formed new zirconocene heterocycles.
[14] A special feature of titanocene bis(trimethylsilyl) and its zirconium analogues is the ability it derives from coordination of the alkyne to stabilize the metallocene fragment.
For a comprehensive review, visit "Recent Synthetic and Catalytic Applications of Group 4 Metallocene Bis(trimethylsilyl)acetylene Complexes".
[36] As main side products of coupling reactions with Rosenthal's reagent, pyridine and bis(trimethylsilyl)acetylene are obtained.
[37] Past synthesis, including those mentioned previously, have been straightforward, but require extreme caution in the exclusion of water and air to obtain a pure, catalytically useful complex.
[40] Similarly, the synthesis of other titanocene bis(trimethylsilyl) acetylene complexes have been reported, such as the low-valent ansa-dimethylsilylene, dimethylmethylene–bis(cyclopentadienyl)titanium.
[43] Later, Vol’pin again utilized the isolobal analogy to react diphenylacetylene with titanocene (Cp2Ti, where Cp = cyclopentadienyl, rather than dialkylsilene) in an attempt to synthesize unsaturated 1-heterocyclopropanes.