Roskamp reaction
According to its resonance structure, the carbon adjacent to the diazo group has partial negative charge.
[6] Olefins can be used to generate the desired aldehyde in situ through ozonolysis, where tin(II) chloride would serve as both the reducing agent in the ozonolysis step as well as the Lewis acid catalyst in the Roskamp reaction step.
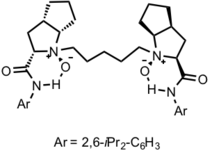
In 2009, Maruoka and co-workers reported a Lewis acid-catalyzed asymmetric Roskamp reaction.
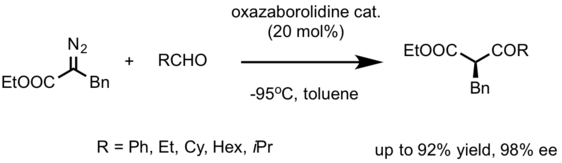
In 2012, Do Hyun Ryu from Sungkyunkwan University developed a catalytic, asymmetric Roskamp reaction with broad applicability.
In 2015, the same group reported asymmetric Roskamp reaction of the α-aryl diazo Weinreb amide, using the same chiral oxazaborolidine catalyst.