RuBisCO
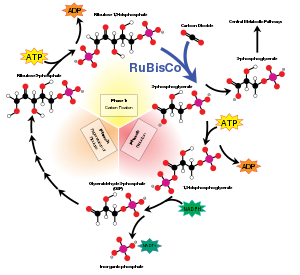
[5][6][7] RuBisCO is important biologically because it catalyzes the primary chemical reaction by which inorganic carbon enters the biosphere.
The role of changing pH and magnesium ion levels in the regulation of RuBisCO enzyme activity is discussed below.
[13] This gradient is established by the dimer form of the minimally active RuBisCO, which with its two components provides a combination of oppositely charged domains required for the enzyme's interaction with O2 and CO2.
These conditions help explain the low turnover rate found in RuBisCO: In order to increase the strength of the electric field necessary for sufficient interaction with the substrates’ quadrupole moments, the C- and N- terminal segments of the enzyme must be closed off, allowing the active site to be isolated from the solvent and lowering the dielectric constant.
[20][21] Carboxylation of the 2,3-enediolate results in the intermediate 3-keto-2-carboxyarabinitol-1,5-bisphosphate and Lys334 is positioned to facilitate the addition of the CO2 substrate as it replaces the third Mg2+-coordinated water molecule and add directly to the enediol.
Within the spinach structure, other residues are well placed to aid in the hydration step as they are within hydrogen bonding distance of the water molecule.
[14] When carbon dioxide is the substrate, the product of the carboxylase reaction is an unstable six-carbon phosphorylated intermediate known as 3-keto-2-carboxyarabinitol-1,5-bisphosphate, which decays rapidly into two molecules of glycerate-3-phosphate.
Phosphoglycolate is recycled through a sequence of reactions called photorespiration, which involves enzymes and cytochromes located in the mitochondria and peroxisomes (this is a case of metabolite repair).
Nevertheless, under most conditions, and when light is not otherwise limiting photosynthesis, the speed of RuBisCO responds positively to increasing carbon dioxide concentration.
In addition, the activity of RuBisCO is coordinated with that of the other enzymes of the Calvin cycle in several other ways: Upon illumination of the chloroplasts, the pH of the stroma rises from 7.0 to 8.0 because of the proton (hydrogen ion, H+) gradient created across the thylakoid membrane.
[26][27] This is required because ribulose 1,5-bisphosphate (RuBP) binds more strongly to the active sites of RuBisCO when excess carbamate is present, preventing processes from moving forward.
Furthermore, in most plants, the sensitivity of activase to the ratio of ATP/ADP is modified by the stromal reduction/oxidation (redox) state through another small regulatory protein, thioredoxin.
This weakness in the enzyme is the cause of photorespiration, such that healthy leaves in bright light may have zero net carbon fixation when the ratio of O2 to CO2 available to RuBisCO shifts too far towards oxygen.
The process first makes a 4-carbon intermediate compound, hence the name C4 plants, which is shuttled into a site of C3 photosynthesis then decarboxylated, releasing CO2 to boost the concentration of CO2.
Crassulacean acid metabolism (CAM) plants keep their stomata closed during the day, which conserves water but prevents the light-independent reactions (a.k.a.
[42] Robust and reliable engineering for yield of RuBisCO and other enzymes in the C3 cycle was shown to be possible,[43] and it was first achieved in 2019 through a synthetic biology approach.
[37] One avenue is to introduce RuBisCO variants with naturally high specificity values such as the ones from the red alga Galdieria partita into plants.
[46] A recent theory explores the trade-off between the relative specificity (i.e., ability to favour CO2 fixation over O2 incorporation, which leads to the energy-wasteful process of photorespiration) and the rate at which product is formed.
[47] It has been also suggested that the oxygenase reaction of RuBisCO prevents CO2 depletion near its active sites and provides the maintenance of the chloroplast redox state.
There currently are very few effective methods for expressing functional plant Rubisco in bacterial hosts for genetic manipulation studies.
This is largely due to Rubisco's requirement of complex cellular machinery for its biogenesis and metabolic maintenance including the nuclear-encoded RbcS subunits, which are typically imported into chloroplasts as unfolded proteins.
[54] Other existing methods for depleting RuBisCO and studying lower abundance proteins include fractionation techniques with calcium and phytate,[55] gel electrophoresis with polyethylene glycol,[56][57] affinity chromatography,[58][59] and aggregation using DTT,[60] though these methods are more time-consuming and less efficient when compared to protamine sulfate precipitation.
[53] The chloroplast gene rbcL, which codes for the large subunit of RuBisCO has been widely used as an appropriate locus for analysis of phylogenetics in plant taxonomy.
It is now believed that the current RuBisCO evolved from a dimeric RLP ancestor, acquiring its carboxylase function first before further oligomerizing and then recruiting the small subunit to form the familiar modern enzyme.
[15] The small subunit probably first evolved in anaerobic and thermophilic organisms, where it enabled RuBisCO to catalyze its reaction at higher temperatures.
Laboratory-based phylogenetic studies have shown that this evolution was constrained by the trade-off between stability and activity brought about by the series of necessary mutations for C4 RuBisCO.
The destabilizing C4 mutations on RuBisCO has been sustained by environmental pressures such as low CO2 concentrations, requiring a sacrifice of stability for new adaptive functions.
[65] The term "RuBisCO" was coined humorously in 1979, by David Eisenberg at a seminar honouring the retirement of the early, prominent RuBisCO researcher, Sam Wildman, and also alluded to the snack food trade name "Nabisco" in reference to Wildman's attempts to create an edible protein supplement from tobacco leaves.
It can be capitalized for each letter of the full name (Ribulose-1,5 bisphosphate carboxylase/oxygenase), but it has also been argued that is should all be in lower case (rubisco), similar to other terms like scuba or laser.