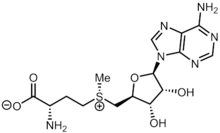
S-Adenosyl methionine
S-Adenosyl methionine (SAM), also known under the commercial names of SAMe, SAM-e, or AdoMet, is a common cosubstrate involved in methyl group transfers, transsulfuration, and aminopropylation.
In eukaryotic cells, SAM serves as a regulator of a variety of processes including DNA, tRNA, and rRNA methylation; immune response;[2] amino acid metabolism; transsulfuration; and more.
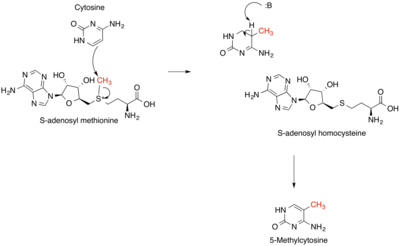
In the first step of this cycle, the SAM-dependent methylases (EC 2.1.1) that use SAM as a substrate produce S-adenosyl homocysteine as a product.
[7] Most enzymes with this capability share a region of sequence homology that includes the motif CxxxCxxC or a close variant.
Examples of radical SAM enzymes include spore photoproduct lyase, activases of pyruvate formate lyase and anaerobic sulfatases, lysine 2,3-aminomutase, and various enzymes of cofactor biosynthesis, peptide modification, metalloprotein cluster formation, tRNA modification, lipid metabolism, etc.
[10][11] As of 2012, the evidence was inconclusive as to whether SAM can mitigate the pain of osteoarthritis; clinical trials that had been conducted were too small from which to generalize.
"[16] A 2020 systematic review found that it performed significantly better than placebo, and had similar outcomes to other commonly used antidepressants (imipramine and escitalopram).
In mouse models, excess levels of SAM have been implicated in erroneous methylation patterns associated with diabetic neuropathy.
In vitro addition in such cancers has been shown to remethylate oncogene promoter sequences and decrease the production of proto-oncogenes.
Contrary to the former information, colorectal cancers (CRCs) are characterized by global hypomethylation and promoter-specific DNA methylation.
[21] In Canada, the UK,[22] and the United States, SAM is sold as a dietary supplement under the marketing name SAM-e (also spelled SAME or SAMe).
These drugs include, but are certainly not limited to, dextromethorphan (Robitussin), meperidine (Demerol), pentazocine (Talwin), and tramadol (Ultram).
Jean-Michel Fustin of Manchester University said that the researchers found that excess SAMe breaks down into adenine and methylthioadenosine in the body, both producing the paradoxical effect of inhibiting methylation.