Isatin
[5] It looks like a red-orange powder, and it is usually employed as building block for the synthesis of a wide variety of biologically active compounds including antitumorals,[6] antivirals,[7] anti-HIVs,[8] and antituberculars.
[11] The method involves the condensation between chloral hydrate and a primary arylamine (e.g. aniline), in the presence of hydroxylamine hydrochloride, in aqueous sodium sulfate to form an α‐isonitrosoacetanilide.
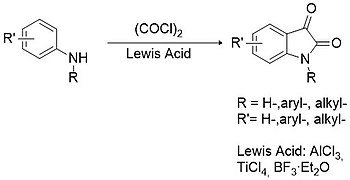
[12] In this case primary or secondary arylamines are condensed with oxalyl chloride to form a chlorooxalylanilide intermediate which can then cyclize in the presence of a Lewis acid (e.g. aluminium trichloride, titanium tetrachloride, boron trifluoride, etc.
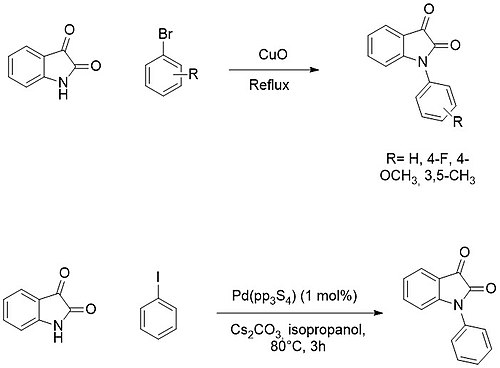
The N-functionalization of the isatin core can be readily obtained by the deprotonation of the amino moiety, forming the corresponding sodium or potassium salt, and subsequent addition of an electrophile (e.g. alkyl or acyl halides).
[19] In another one-pot multicomponent reaction, a unique two-carbon expansion has been achieved by reacting isatin with indene-1,3-dione and N-substituted pyridinium bromide to form dibenzo[b,d]azepin-6-ones.
The regioselectivity of the process strongly depends both on the substrate (properties of the substituents on the isatin core, especially those bonded to the nitrogen atom) and the reaction conditions (solvent, temperature etc.).