Scanning electrochemical microscopy
[15] SECM continues to increase in popularity due to theoretical and technological advances that expand experimental modes while broadening substrate scope and enhancing sensitivity.
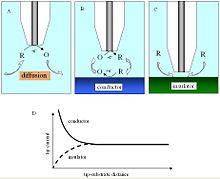
where iT,∞ is the diffusion-limited current, n is the number of electrons transferred at the electrode tip (O + ne− → R), F is Faraday's constant, C is the concentration of the oxidized species in solution, D is the diffusion coefficient and a is the radius of the UME disc.
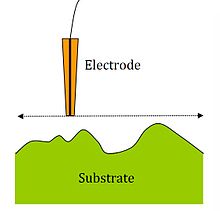
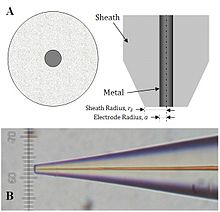
An additional parameter to consider when probing insulating surfaces is the electrode sheath diameter, rg, since it contributes to the physical obstruction of diffusion.
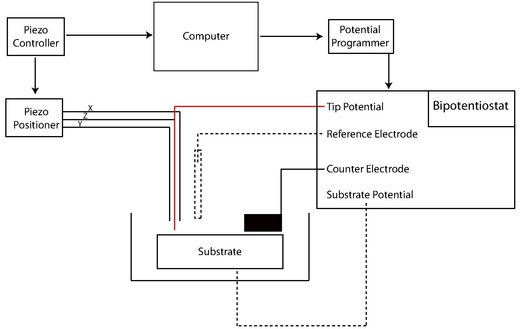
Early SECMs were constructed solely by individual lab groups from a set of common components including potentiostat (or bipotentiostat) and potential programmer, current amplifier, piezoelectric positioner and controller, computer, and UME.
The development of new techniques toward the reliable nanofabrication of electrodes has been a primary focus in the literature due to several distinct advantages including high mass-transfer rates and low levels of reactant adsorption in kinetic experiments.
[23][24] Additionally, enhanced spatial resolution afforded by reduced tip size expands the scope of SECM studies to smaller and faster phenomena.
Etched metal wires can then be coated with wax, varnish, molten paraffin or glass, poly(a-methylstyrene), polyimide,[26] electropolymerized phenol, and electrophoretic paint.
"Penetration" experiments, where the tip is inserted into a microstructure (such as a thin polymer film with fixed redox centers) to probe kinetic and concentration parameters, also require the use of nanoscale electrodes.
SECM-AFM probes can act as both a force sensor and electrode through the utilization of a flattened, etched metal wire coated by electrophoretic paint.
[2] Similarly, SECM functionality can be imparted into standard AFM probes by sputtering the surface with a conductive metal or by milling an insulated tip with a focused ion beam (FIB).
"Soft stylus probes" were recently developed by filling a microfabricated track on a polyethylene terephthalate sheet with a conductive carbon ink.
Scanning electron microscopy (SEM), cyclic voltammetry (CV), and SECM approach curve measurements are frequently applied to identify the dimension and geometry of fabricated probes.
More specifically, systems may feature an inchworm motor that directs coarse positioning with additional z control governed by a PZT piezo pusher.
[15] SECM has been employed to probe the topography and surface reactivity of solid-state materials, track the dissolution kinetics of ionic crystals in aqueous environments, screen electrocatalytic prospects, elucidate enzymatic activities, and investigate dynamic transport across synthetic/natural membranes and other biophysical systems.
An early example demonstrated patterning of dodecylthiolate self-assembled monolayers (SAMs) by moving the UME in a two-dimensional array in close proximity to the surface while applying an oxidative or reductive potential, thus locally desorbing the chemical species.
An inherent benefit of SECM over other SPL techniques for surface patterning can be attributed to its ability to simultaneously acquire surface-related electrochemical information while performing lithography.
Other studies have demonstrated the utility of SECM for the deposition of local gold islands as templates for attachment of biomolecules and fluorescent dyes.
[34] Here, glass microcapillaries with sub-micron sized orifices replace the standard UME allowing femtoliter-sized droplets to be suspended from the capillary over a conductive surface acting as the working electrode.
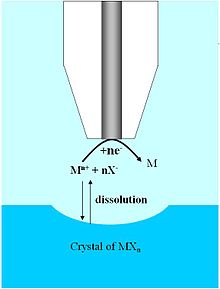
The dissolution of ionic crystals in aqueous environments is fundamentally important to the characterization of a host of naturally occurring and synthetic systems.
[35] The high spatial resolution and three-dimensional mobility provided by the UME allows one to probe the dissolution kinetics on specific faces of single ionic crystals, whereas previous characterization techniques relied on a bulk or ensemble average measurement.
Due to the high mass transfer rates associated with UMEs in the SECM configuration, it is possible to quantify systems defined by very fast reaction kinetics.
In addition, UMEs allow monitoring over a wide dynamic range, making possible the study of ionic solids with large differences in solubility.
Early examples demonstrating the utility of SECM to extract quantitative rate data from such systems was carried out on CuSO4 crystals in an aqueous solution saturated with Cu2+ and SO2−4 ions.
Some groups studying electrocatalysis have demonstrated the use of SECM as a rapid screening technique that provides local quantitative electrochemical information about catalytic mixtures and materials.
One functional, non-SECM approach, enabled the electrocatalytic activities of a large number of catalysts to be assessed optically by employing a technique that detected proton production on deposited arrays of proton-sensitive fluorescent dyes.
Such high throughput screening significantly assists the search for abundant, efficient and cost-effective electrocatalytic materials as substitutes for platinum and other precious metals.
The ability to probe non-conductive surfaces makes SECM a feasible method for analyzing membranes, redox active enzymes, and other biophysical systems.
Redox processes of individual living cells can be probed by SECM, which serves as a non-invasive method for monitoring intracellular charge transfer.
In such measurements, the cell of interest is immobilized on a surface submerged in a solution with the oxidized form of the redox mediator and feedback mode is employed.
A study by Liu et al.[41] employed this method and showed that the redox states within three human breast cell lines (nonmotile, motile, and metastatic) were consistently different.