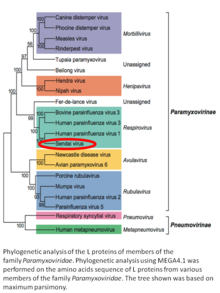
Murine respirovirus
SeV has several features that are important in a vector for a successful vaccine: the virus does not integrate into the host genome, it does not undergo genetic recombination, it replicates only in the cytoplasm without DNA intermediates or a nuclear phase and it does not cause any disease in humans or domestic animals.
Imported animals should be vaccinated with SeV and placed in quarantine, while, in the laboratory environment, breeding programs should be discontinued, and the non-breeding adults isolated for two months.
[59] Currently, there is no scientific data obtained using modern detection methods that would identify SeV as an infectious - disease causing agent for humans or domestic animals.
After experimental SeV infection the virus can replicate and shed from the upper and lower respiratory tract of African green monkeys and chimpanzees, but it is not causing any disease.
[114] In addition, SeV triggers the expression of the chemokine interferon-γ inducible protein 10 kDa (CXCL10), which is involved in chemotaxis, induction of apoptosis, regulation of cell growth and mediation of angiostatic effects.
[124] In response to SeV infection, the production of hBD-1 mRNA and protein increases 2 hours after exposure to the virus in purified plasmacytoid dendritic cells or in PBMC.
Moreover, even the animals that are unresponsive to type I IFN develop long-term anti-SeV immunity in a form of memory response that includes generation of CD8+ T cells and neutralizing antibodies.
Evaluation of the safety and immunogenicity of an intranasally administered replication-competent Sendai Virus–vectored HIV Type 1 gag vaccine demonstrated: induction of potent T-Cell and antibody responses in prime-boost regimens.
[9] Short-term remission after an intravenous injection of SeV was described in a patient with acute leukemia treated in the Clinical Research Center of University Hospitals of Cleveland (USA) by multiple viruses in 1964.
[145] It is also reported[8][146] that the Moscow strain of SeV[147] was tested by Dr. V. Senin[148] and his team as an anticancer agent in a few dozen patients affected by various malignancies with metastatic growth in Russia in the 1990s.
[149] Intratumoral injection of UV irradiated and inactivated SeV resulted in an antitumor effect in a few melanoma patients with stage IIIC or IV progressive disease with skin or lymph metastasis.
[159][160] Among other receptors represented by gangliosides GT1b is highly expressed on the outer membranes of brain metastases cells that originate from an extremely broad range of cancer,[161] while GD1a,[156] GT1b[162] and GQ1b[163] can be detected in human gliosarcomas.
[251] NA also promotes cell fusion, which helps the nascent virions to avoid contact with host antibodies and thus enables the virus to spread within tissues.
Therefore, the ability of SeV sialidase (NA) to remove sialic acid from the surface of malignant cells most likely helps to ensure the availability of tumor antigens for recognition by cytotoxic T lymphocytes.
This was shown in a mouse model of renal cancer, in which the anti-tumor effect of SeV was suppressed by reducing the number of NK cells by co-injection of specific antibodies.
First, the functional hemagglutinin-neuraminidase protein of the oncolytic Newcastle disease virus (NDV), which is a relative of SeV, has been shown to enhance the tumor-specific cytotoxic response of CD8+ T-cells and to increase the activity of CD4+ T-helper cells.
[108] SeV has been known to the research community since the late 1950s and has been widely used to create numerous variants of genetically engineered constructs, including vectors for transgene delivery.
Due to SeV genetic stability, multiple serial passages of the virus construct in cell cultures or embryonated chicken eggs without drastic genomic changes are possible.
The recovery and amplification of SeV/ΔF vectors proceed as follows: Researchers have developed an innovative method to efficiently produce substantial quantities of heterologous viral glycoproteins within the allantoic cavity of embryonated chicken eggs.
[308] SeV has several features that are important in a vector for a successful vaccine: the virus does not integrate into the host genome, it does not undergo genetic recombination, it replicates only in the cytoplasm without DNA intermediates or a nuclear phase.
The study that was published in 2011 demonstrated that SeV neutralizing antibodies (which were formed due to HPIV-1 past infection) can be detected in 92.5% subjects worldwide with a median EC50 titer of 60.6 and values ranging from 5.9–11,324.
Evaluation of the safety and immunogenicity of an intranasally administered replication-competent Sendai Virus–vectored HIV Type 1 gag vaccine demonstrated: induction of potent T-Cell and antibody responses in prime-boost regimens.
[15][312][313] For effective prevention of infections caused by SARS-CoV-2, the ability of the vaccine to stimulate the mucosal immunity of the upper respiratory tract, including the nasal cavity, might be highly important.
One design utilizes a modified Sendai virus (SeV) as a vector to deliver the SARS-CoV-2 spike protein's receptor binding domain (RBD) directly to the respiratory tract.
Mice demonstrated elevated levels of antibodies specific to the SARS-CoV-2 S-RBD (IgM, IgG, IgA) in both their blood serum and bronchoalveolar lavage fluid, lasting up to 12 weeks.
[159][160] Among other receptors represented by gangliosides GT1b is highly expressed on the outer membranes of brain metastases cells that originate from an extremely broad range of cancer,[161] while GD1a,[156] GT1b[162] and GQ1b[163] can be detected in human gliosarcomas.
[380] Two of SeV proteins: HA and F, after their binding directly to a cellular membrane, promote a cell-cell fusion, which leads to a large multinuclear cell formation (syncytium).
Thus, a SeV infection in a form of genetic material in partially assembled virions can spread without any exposure to host neutralizing antibodies (see the section "Directed cells fusion (syncytium formation)" for details and references).
[389] One recognized feature of the Sendai virus, shared with members of its genus, is the ability to induce syncytia formation in vivo and in vitro in eukaryotic cell cultures.
[411][412] A single amino acid substitution in a nucleoprotein (NP) causes an increased production rate of DI genomes in the SeV Cantell strain, which is known for its particularly strong induction of interferon beta (IFN-β) during viral infection.




Case 1. Male dog of 7 years old developed cutaneous, ulcerated, and poorly differentiated mastocytoma (35 mm diameter) located close to his anus. (1) Primary tumor; (2) 2 weeks after the first virus treatment; (3) 4 weeks after the first virus treatment.
Case 2. Male German shorthaired pointer of 9 years old developed subcutaneous, regional (stage 2) intermediately differentiated mastocytoma. The primary tumor was removed without clean margins. (1) secondary growth 1 week after the surgical procedure; (2) 2 weeks after the first virus treatment; (3) 5 weeks after the first virus treatment.










The positions of translation initiation sites for products of the alternative reading frame of the P-coding mRNA