Sigmatropic reaction
An odd number is an indication of the involvement of a charged C atom or of a heteroatom lone pair replacing a CC double bond.
The numbers that correspond to the atoms forming the new bond are then separated by a comma and placed within brackets to create the sigmatropic reaction order descriptor.
For a sigmatropic reaction, the transition state will consist of two fragments, joined by the forming and breaking σ-bonds.
[4] In cases of stereochemical retention, the migrating group translates without rotation into the bonding position, while in the case of stereochemical inversion the migrating group both rotates and translates to reach its bonded conformation.
However, another stereochemical transition effect equally capable of producing inversion or retention products is whether the migrating group remains on the original face of the π system after rebonding or instead transfers to the opposite face of the π system.
If the migrating group remains on the same face of the π system, the shift is known as suprafacial, while if the migrating group transfers to the opposite face is called an antarafacial shift,[3] which are impossible for transformations that occur within small- or medium-sized rings.
Here the geometry of the transition state is prohibitive, but an alkyl group, due to the nature of its orbitals, can invert its geometry, form a new bond with the back lobe of its sp3 orbital, and therefore proceed via a suprafacial shift.
These reactions are still not common in open-chain compounds because of the highly ordered nature of the transition state, which is more readily achieved in cyclic molecules.
[1,7] sigmatropic shifts are predicted by the Woodward–Hoffmann rules to proceed in an antarafacial fashion, via a Mobius topology transition state.
The Woodward–Hoffman rules predict that these six-electron reactions would proceed suprafacially, via a Hückel topology transition state.
The formation of a carbonyl group makes this reaction, unlike other sigmatropic rearrangements, inherently irreversible.
The ortho-Claisen rearrangement involves the [3,3] shift of an allyl phenyl ether to an intermediate which quickly tautomerizes to an ortho-substituted phenol.
[18] This organic reaction is accompanied by decarboxylation and the final product is a γ,δ-allylketone.
When heated, the pi-system goes through an electrocyclic ring closing to form bicycle[4,1,0]heptadiene (norcaradiene).

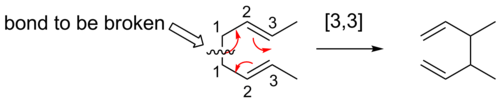





![[1,3] Alkyl shifts](http://upload.wikimedia.org/wikipedia/commons/thumb/e/e9/1%2C3alkylfixed.png/550px-1%2C3alkylfixed.png)
![[1,5] hydride shift in a cyclic system](http://upload.wikimedia.org/wikipedia/commons/thumb/7/7f/1%2C5hydridecyclicfixed.png/300px-1%2C5hydridecyclicfixed.png)
![Antarafacial [1,5] hydride shift](http://upload.wikimedia.org/wikipedia/commons/thumb/9/99/1%2C5hantarafacialfixed.png/600px-1%2C5hantarafacialfixed.png)




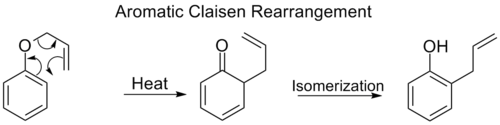


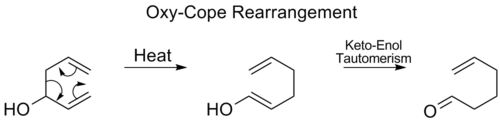


![[5,5] shift of phenyl pentadienyl ether](http://upload.wikimedia.org/wikipedia/commons/thumb/2/25/5%2C5shiftfixeds.png/800px-5%2C5shiftfixeds.png)
