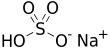Sodium bisulfate
It is also used in metal finishing, cleaning products,[2] and to lower the pH of water for effective chlorination in swimming pools and hot tubs.
[7] In the textiles industry, it is sometimes applied to velvet cloth made with a silk backing and a pile of cellulose-based fiber (rayon, cotton, hemp, etc.)
to create "burnout velvet": the sodium bisulfate, when applied to such a fabric and heated, causes the cellulose-based fibers to become brittle and flake away, leaving burned-out areas in the finished material, usually in attractive patterns.
[10] Sodium bisulfate is sometimes used as the active ingredient in flocculant tablets, a step in soil and water quality test kits.
[11] Sodium bisulfate is used as a food additive to leaven cake mixes (make them rise)[12] as well as being used in meat and poultry processing and most recently in browning prevention of fresh-cut produce.
Sodium bisulfate lowers the pH without creating a sour taste, and has been used in the place of citric, malic, or phosphoric acids that are commercially available,[9] and it can also be used as an anti-browning agent.