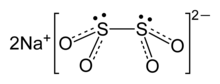
Sodium dithionite
[2] Because this bond is fragile, the dithionite anion dissociates in solution into the [SO2]− radicals, as has been confirmed by EPR spectroscopy.
It is also observed that 35S undergoes rapid exchange between S2O42− and SO2 in neutral or acidic solution, consistent with the weak S-S bond in the anion.
Sodium dithionite is stable when dry, but aqueous solutions deteriorate due to the following reaction: This behavior is consistent with the instability of dithionous acid.
[8] Sodium dithionite can also be used for water treatment, aquarium water conditioners, gas purification, cleaning, and stripping.In addition to the textile industry, this compound is used in industries concerned with leather, foods, polymers, photography, and many others, often as a decolourising agent.
It is even used domestically as a decoloring agent for white laundry, when it has been accidentally stained by way of a dyed item slipping into the high temperature washing cycle.
[9] Sodium dithionite is often used in physiology experiments as a means of lowering solutions' redox potential (Eo' -0.66 V vs SHE at pH 7).
In addition, sodium dithionite is often used in soil chemistry experiments to determine the amount of iron that is not incorporated in primary silicate minerals.
[11] Pyrithione can be prepared in a two-step synthesis from 2-bromopyridine by oxidation to the N-oxide with a suitable peracid followed by substitution using sodium dithionite to introduce the thiol functional group.