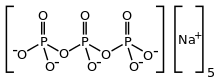
Sodium triphosphate
[4][5] Many related di-, tri-, and polyphosphates are known including the cyclic triphosphate (e.g. sodium trimetaphosphate).
Being a highly charged chelating agent, TPP5− binds to dications tightly and prevents them from interfering with the sulfonate detergent.
Many governments regulate the quantities allowed in foods, as it can substantially increase the sale weight of seafood in particular.
The United States Food and Drug Administration lists STPP as Generally recognized as safe.
[15] Salts of polyphosphate anions are moderately irritating to skin and mucous membranes because they are mildly alkaline.