Sodium tungsten bronze
[2] A similar family of molybdenum bronzes may have been discovered in 1885 by Alfred Stavenhagen and E. Engels,[3] but they are formed in a very narrow range of temperatures and were not reported again until the 1960s.
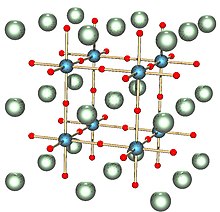
Colour is dependent upon the proportion of sodium in the compound, ranging from golden at x ≈ 0.9, through red, orange and deep purple, to blue-black when x ≈ 0.3.
[5] It has been suggested that electrons, released when the sodium atoms are ionised, are conducted readily through the tungsten t2g and oxygen π orbitals.
[2] When cooled sufficiently, sodium tungsten bronze becomes a superconductor, with the critical temperature (Tc) for Na0.23WO3 being approximately 2.2 kelvin.
[11] Wöhler's 1823 synthesis involved reducing sodium tungstate and tungsten trioxide with hydrogen gas at red heat.