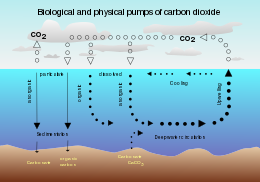
Solubility pump
The solubility pump is driven by the coincidence of two processes in the ocean : Since deep water (that is, seawater in the ocean's interior) is formed under the same surface conditions that promote carbon dioxide solubility, it contains a higher concentration of dissolved inorganic carbon than might be expected from average surface concentrations.
One consequence of this is that when deep water upwells in warmer, equatorial latitudes, it strongly outgasses carbon dioxide to the atmosphere because of the reduced solubility of the gas.
However, unlike many other gases (oxygen for instance), it reacts with water and forms a balance of several ionic and non-ionic species (collectively known as dissolved inorganic carbon, or DIC).
Presently, about one third (approximately 2 gigatons of carbon per year)[2][3] of anthropogenic emissions of CO2 are believed to be entering the ocean.
The solubility pump is the primary mechanism driving this flux, with the consequence that anthropogenic CO2 is reaching the ocean interior via high latitude sites of deep water formation (particularly the North Atlantic).
However, the magnitude of these processes is still uncertain, preventing good long-term estimates of the fate of the solubility pump.