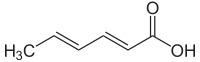
Sorbic acid
[2] It can also be prepared from isomeric hexadienoic acids, which are available via a nickel-catalyzed reaction of allyl chloride, acetylene, and carbon monoxide.
Beginning in the 1980s, sorbic acid and its salts were used as inhibitors of Clostridium botulinum in meat products to replace the use of nitrites, which can produce carcinogenic nitrosamines.
It is found in foods such as various kinds of cheese, bread, muffins, donuts, pies, cookies, protein bars, syrups, lemonades, fruit juices, dried meats, sausages, nuggets, burgers, sandwiches, tacos, pizzas, smoked fish, margarine, sauces, soups, and more.
[6] The E numbers are: Some molds (notably some Trichoderma and Penicillium strains) and yeasts are able to detoxify sorbates by decarboxylation, producing trans-1,3-pentadiene.
[7] Sorbic acid can also be used as an additive for cold rubber, and as an intermediate in the manufacture of some plasticizers and lubricants.