Spin isomers of hydrogen
When hydrogen is liquified at low temperature, there is a slow spontaneous transition to a predominantly para ratio, with the released energy having implications for storage.
If we neglect the small proportion of deuterium and tritium which may be present, each hydrogen atom consists of one proton and one electron.
The triplet orthohydrogen state has total nuclear spin I = 1 so that the component along a defined axis can have the three values MI = 1, 0, or −1.
[1] Since protons have spin 1⁄2, they are fermions and the permutational antisymmetry of the total H2 wavefunction imposes restrictions on the possible rotational states of the two forms of H2.
As a result, ortho liquid hydrogen equilibrating to the para form releases enough energy to cause significant loss by boiling.
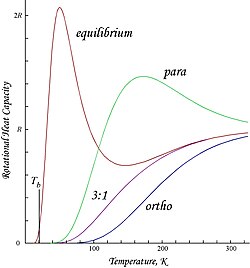
[6] Applying the rigid rotor approximation, the energies and degeneracies of the rotational states are given by:[9][page needed] The rotational partition function is conventionally written as:[citation needed] However, as long as the two spin isomers are not in equilibrium, it is more useful to write separate partition functions for each:[citation needed] The factor of 3 in the partition function for orthohydrogen accounts for the spin degeneracy associated with the +1 spin state; when equilibrium between the spin isomers is possible, then a general partition function incorporating this degeneracy difference can be written as:[citation needed] The molar rotational energies and heat capacities are derived for any of these cases from:[citation needed] Plots shown here are molar rotational energies and heat capacities for ortho- and parahydrogen, and the "normal" ortho:para ratio (3:1) and equilibrium mixtures:[citation needed] Because of the antisymmetry-imposed restriction on possible rotational states, orthohydrogen has residual rotational energy at low temperature wherein nearly all the molecules are in the J = 1 state (molecules in the symmetric spin-triplet state cannot fall into the lowest, symmetric rotational state) and possesses nuclear-spin entropy due to the triplet state's threefold degeneracy.
Since "normal" room-temperature hydrogen is a 3:1 ortho:para mixture, its molar residual rotational energy at low temperature is (3/4) × 2Rθrot ≈ 1091 J/mol,[citation needed] which is somewhat larger than the enthalpy of vaporization of normal hydrogen, 904 J/mol at the boiling point, Tb ≈ 20.369 K.[10] Notably, the boiling points of parahydrogen and normal (3:1) hydrogen are nearly equal; for parahydrogen ∆Hvap ≈ 898 J/mol at Tb ≈ 20.277 K, and it follows that nearly all the residual rotational energy of orthohydrogen is retained in the liquid state.
[11] This process lacks any natural de-excitation radiation mode, so it is slow in the absence of a catalyst which can facilitate interconversion of the singlet and triplet spin states.
[11] At room temperature, hydrogen contains 75% orthohydrogen, a proportion which the liquefaction process preserves if carried out in the absence of a catalyst like ferric oxide, activated carbon, platinized asbestos, rare earth metals, uranium compounds, chromic oxide, or some nickel compounds to accelerate the conversion of the liquid hydrogen into parahydrogen.
[citation needed] If orthohydrogen is not removed from rapidly liquified hydrogen, without a catalyst, the heat released during its decay can boil off as much as 50% of the original liquid.
Taking into account this theoretical framework, pure parahydrogen was first synthesized by Paul Harteck and Karl Friedrich Bonhoeffer in 1929 at the Kaiser Wilhelm Institute for Physical Chemistry and Electrochemistry.
[13][14] When Heisenberg was awarded the 1932 Nobel prize in physics for the creation of quantum mechanics, this discovery of the "allotropic forms of hydrogen" was singled out as its most noteworthy application.
[15] Further work on the properties and chemical reactivity of parahydrogen was carried out in the following decade by Elly Schwab-Agallidis and Georg-Maria Schwab.
[20][21] Signal amplification by reversible exchange (SABRE) is a technique to hyperpolarize samples without chemically modifying them.
Compared to orthohydrogen or organic molecules, a much greater fraction of the hydrogen nuclei in parahydrogen align with an applied magnetic field.
[25] This enhanced NMR signal allows the rapid analysis of very small amounts of material and has great potential for applications in magnetic resonance imaging.
[26] There are six possible nuclear spin wave functions which are ortho or symmetric to exchange of the two nuclei, and three which are para or antisymmetric.
[26] Other molecules and functional groups containing two hydrogen atoms, such as water[27] and methylene (CH2),[28] also have ortho- and para- forms (e.g. orthowater and parawater), but this is of little significance for their thermal properties.