Stress corrosion cracking
It can lead to unexpected and sudden failure of normally ductile metal alloys subjected to a tensile stress, especially at elevated temperature.
The specific environment is of crucial importance, and only very small concentrations of certain highly active chemicals are needed to produce catastrophic cracking, often leading to devastating and unexpected failure.
Unexpected and premature failure of chemical process equipment, for example, due to stress corrosion cracking constitutes a serious hazard in terms of safety of personnel, operating facilities and the environment.
A comparable effect also known as environmental stress cracking also affects other materials such as polymers, ceramics and glass.
A similar process (environmental stress cracking) occurs in polymers, when products are exposed to specific solvents or aggressive chemicals such as acids and alkalis.
Polymers are susceptible to environmental stress cracking where attacking agents do not necessarily degrade the materials chemically.
For example, the fracture surface of a fuel connector showed the progressive growth of the crack from acid attack (Ch) to the final cusp (C) of polymer.
In this case the failure was caused by hydrolysis of the polymer by contact with sulfuric acid leaking from a car battery.
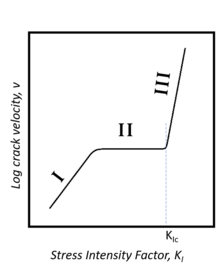
In region I, the velocity of crack propagation increases with ambient humidity due to stress-enhanced chemical reaction between the glass and water.
In region III, crack propagation is independent of its environment, having reached a critical stress intensity.