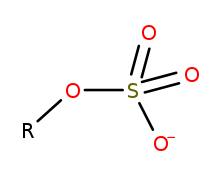
Organosulfate
In organosulfur chemistry, organosulfates are a class of organic compounds sharing a common functional group with the structure R−O−SO−3.
[2] Alkylsulfate can be produced from alcohols, which in turn are obtained by hydrogenation of animal or vegetable oils and fats or using the Ziegler process or through oxo synthesis.
If derived using the oxo process, a low level of branching will appear usually with a methyl or ethyl group at the C-2 position, containing even and odd amounts of alkyl chains.
[8] Sulfate is an inert anion, so nature activates it by the formation of ester derivative of adenosine 5'-phosphosulfate (APS) and 3'-phosphoadenosine-5'-phosphosulfate (PAPS).
Many organisms utilize these reactions for metabolic purposes or for the biosynthesis of sulfur compounds required for life.
The concentration of alkylsulfates in effluent from waste water treatment plants (WWTP) has been measured at 10 micrograms per litre (5.8×10−9 oz/cu in) and lower.
Sodium laurylsulfate tested on Uronema parduczi, a protozoan, was found to have the lowest effect value with the 20 h-EC5 being 0.75 milligrams per litre (2.7×10−8 lb/cu in).
Chronic exposure tests with C12 to C18 with the invertebrate Ceriodaphnia dubia found the highest toxicity is with C14 (NOEC was 0.045 mg/L).
In terms of thermal stability, alkyl sulfates degrade well before reaching their boiling point due to low vapor pressure (for C8-18 from 10-11 to 10-15 hPa).