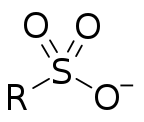
Sulfonate
A classic preparation of sulfonates is the Strecker sulfite alkylation, in which an alkali sulfite salt displaces a halide, typically in the presence of an iodine catalyst:[1] An alternative is the condensation of a sulfonyl halide with an alcohol in pyridine:[2] Esters with the general formula R1SO2OR2 are called sulfonic esters.
Sulfonates are commonly used to confer water solubility to protein crosslinkers such as N-hydroxysulfosuccinimide (Sulfo-NHS), BS3, Sulfo-SMCC, etc.
Some sultones are short-lived intermediates, used as strong alkylating agents to introduce a negatively charged sulfonate group.
In the presence of water, they slowly hydrolyze to the hydroxy sulfonic acids.
Sultone oximes are key intermediates in the synthesis of the anti-convulsant drug zonisamide.