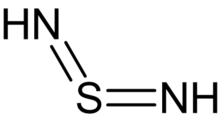
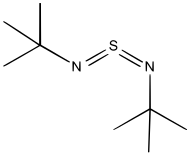
Sulfur diimide
Sulfur diimides are chemical compounds of the formula S(NR)2.
The latter typically requires amide reactants that are less basic than the products,[2] as with disulfonylsulfodiimide... ...or with N,N'-Bis(methoxycarbonyl)sulfur diimide (MeO2C-N=S=N-CO2Me) from methyl carbamate.
[3] Alternatively, the presence of a strong base to absorb the released SO2 can drive transamidation from sulfinylamines.
They have planar C–N=S=N–C cores with bent C–N=S and N=S=N geometries, and various combinations of E and Z isomers are observed for the two N=S bonds.
[1] Organolithium reagents attack at the sulfur to give the corresponding nitrogen anion: The triimido analogues of sulfite can be generated by treating the sulfur diimides with a metal amide:[5] Sulfur diimides undergo Diels–Alder reactions with dienes.