Supercritical fluid extraction
The properties of the supercritical fluid can be altered by varying the pressure and temperature, allowing selective extraction.
[1] The same principle can be used to extract polyphenols and unsaturated fatty acids separately from wine wastes.
[3] The requirement for high pressures increases the cost compared to conventional liquid extraction, so SFE will only be used where there are significant advantages.
Carbon dioxide itself is non-polar, and has somewhat limited dissolving power, so cannot always be used as a solvent on its own, particularly for polar solutes.
Food grade modifiers such as ethanol can often be used, and can also help in the collection of the extracted material, but reduces some of the benefits of using a solvent which is gaseous at room temperature.
This can be either a capillary tube cut to length, or a needle valve which can be adjusted to maintain pressure at different flow rates.
This is problematic if water or other extracted material is present in the sample, as this may freeze in the restrictor or valve and cause blockages.
For analytical SFE, the pressure is usually dropped to atmospheric, and the now gaseous carbon dioxide bubbled through a solvent to trap the precipitated components.
As the fluid is expanded into the separator, heat must be provided to prevent excessive cooling.
For larger systems, the energy required during each stage of the process can be calculated using the thermodynamic properties of the supercritical fluid.
[5] There are two essential steps to SFE, transport (by diffusion or otherwise) of the solid particles to the surface, and dissolution in the supercritical fluid.
Other factors, such as diffusion into the particle by the SF and reversible release such as desorption from an active site are sometimes significant, but not dealt with in detail here.
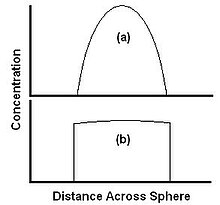
The material is carried away as fast as it arrives at the surface, and the extraction is completely diffusion limited.
The extractant is able to diffuse to the edge faster than it can be carried away by the solvent, and the concentration profile is flat.
In another case, the selectivity may be more important, and a reduced rate of extraction will be preferable if it provides greater discrimination.
This can be achieved by increasing the temperature, swelling the matrix, or reducing the particle size.
Some polymers and elastomers in particular are swelled dramatically by CO2, with diffusion being increased by several orders of magnitude in some cases.
[8] Addition of low levels of modifiers (sometimes called entrainers), such as methanol and ethanol, can also significantly increase solubility, particularly of more polar compounds.
However, to minimize the amount of solvent used, the extraction should be completely solubility limited (which will take a very long time).