Ultrahydrophobicity
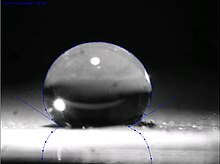
In chemistry and materials science, ultrahydrophobic (or superhydrophobic) surfaces are highly hydrophobic, i.e., extremely difficult to wet.
In 1805, Thomas Young defined the contact angle θ by analysing the forces acting on a fluid droplet resting on a smooth solid surface surrounded by a gas.
By a minimization of free energy argument, the relation that predicted the smaller new contact angle is the state most likely to exist.
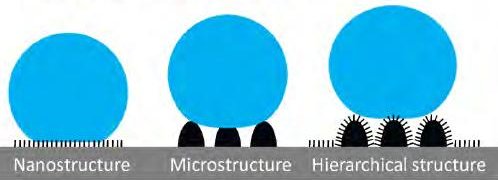
However, in a recent study, Eyal Bittoun and Abraham Marmur found that multiscale roughness is not necessarily essential for superhydrophobicity but beneficial for mechanical stability of the surface.
The fine hairs on some plants are hydrophobic, designed to exploit the solvent properties of water to attract and remove sunlight-blocking dirt from their photosynthetic surfaces.
The skin surface of some species of lizards, such as geckos[22] and anoles,[23] has also been documented as highly hydrophobic, and may facilitate self-cleaning[24] or underwater breathing.
Dettre and Johnson discovered in 1964 that the superhydrophobic lotus effect phenomenon was related to rough hydrophobic surfaces, and they developed a theoretical model based on experiments with glass beads coated with paraffin or TFE telomer.
[26] Perfluoroalkyl, perfluoropolyether and RF plasma formed superhydrophobic materials were developed, used for electrowetting and commercialized for bio-medical applications between 1986 and 1995.
[32] Durable, optically transparent superhydrophobic and oleophobic coatings were developed in 2012 comprising nano particles in the 10 to 100 nm size range.
When the metal cools and solidifies, it is removed from the surface, flipped, and inspected for contact line micro geometry.
[47] The trend shows that as tower width increases, the free energy barrier becomes larger and the contact angle drops, lowering the hydrophobicity of the material.
Droplets naturally move towards areas of weak hydrophobicity, so to make a droplet spontaneously move from one spot to the next, the ideal surface would consist of small width towers with large spacing to large width towers with small spacing.
Initial droplet motion requires an external stimulus, from something as large as a vibration of the surface or as small as a simple syringe "push" as it is released from the needle.
In one study a vanadium pentoxide V2O5 surface is presented that can switch reversibly between superhydrophobicity and superhydrophilicity under the influence of UV radiation.
[49] According to the study any surface can be modified to this effect by application of a suspension of rose-like V2O5 particles for instance with an inkjet printer.
A simple fabrication method could create both microstructure and low surface tension in one step by using octadecyltrichlorosilane (OTS).
[54] Fabricating superhydrophobic polymer surfaces with controlled geometry can be expensive and time consuming, but a small number of commercial sources [citation needed] provide specimens for research labs.
Using silica nano-particles is also of interest to develop transparent hydrophobic materials for car windshields and self-cleaning windows.
An efficient routine has been reported for making linear low-density polyethylene superhydrophobic and thus self-cleaning;[59] 99% of dirt deposited on such a surface is easily washed away.
An example of superhydrophobic effect in live application is the team Alinghi in America's Cup using specially treated sailing jackets.
The resistance to aqueous and organic solvents makes it an ideal choice in developing electronic sensors and chips.
Skin based analyte detection is now possible without damaging and continuous replacing of the electrodes as this paper will be immune to sweat.
A recent application of hydrophobic structures and materials is in the development of micro fuel cell chips.
[62] The membrane consists of many microcavities which allow the gas to escape, while its hydrophobicity characteristic prevents the liquid fuel from leaking through.
[69] In particular, the frost formation over the entire surface is inevitable as a result of undesired inter-droplet freezing wave propagation initiated by the sample edges.
By creating hierarchical surface, the interdroplet freezing wave propagation can be suppressed whereas the ice/frost removal can be promoted.
The enhanced performances are mainly owing to the activation of the microscale edge effect in the hierarchical surface, which increases the energy barrier for ice bridging as well as engendering the liquid lubrication during the deicing/defrosting process.
[70] The ability of packaging to fully empty a viscous liquid is somewhat dependent on the surface energy of the inner walls of the container.