Nucleate boiling
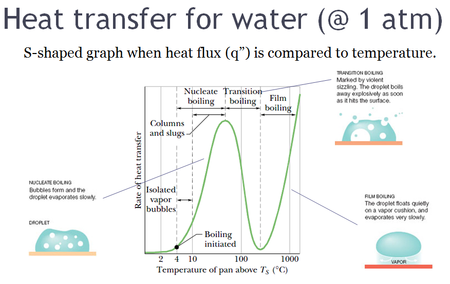
In fluid thermodynamics, nucleate boiling is a type of boiling that takes place when the surface temperature is hotter than the saturated fluid temperature by a certain amount but where the heat flux is below the critical heat flux.
The heat transfer from surface to liquid is greater than that in film boiling.
Two different regimes may be distinguished in the nucleate boiling range.
When the temperature difference is between approximately 4 to 10 °C (7.2 to 18.0 °F) above TS, isolated bubbles form at nucleation sites and separate from the surface.
Interference between the densely populated bubbles inhibits the motion of liquid near the surface.
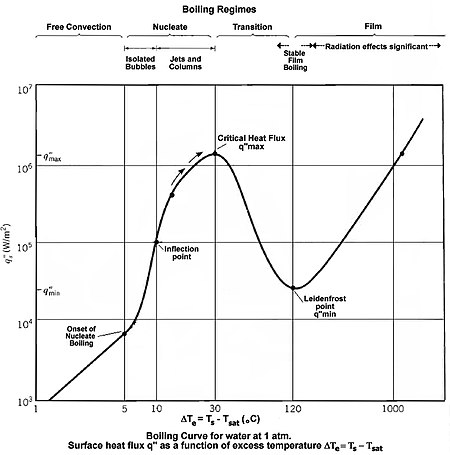
When the relative increase in the temperature difference is balanced by the relative reduction in the heat transfer coefficient, a maximum heat flux is achieved as observed by the peak in the graph.
At extremes, film boiling commonly known as the Leidenfrost effect is observed.
The process of forming steam bubbles within liquid in micro cavities adjacent to the wall if the wall temperature at the heat transfer surface rises above the saturation temperature while the bulk of the liquid (heat exchanger) is subcooled.
The bubbles grow until they reach some critical size, at which point they separate from the wall and are carried into the main fluid stream.
This collapsing is also responsible for the sound a water kettle produces during heat up but before the temperature at which bulk boiling is reached.
This heat transfer process helps quickly and efficiently to carry away the energy created at the heat transfer surface and is therefore sometimes desirable—for example in nuclear power plants, where liquid is used as a coolant.
A limited number of experimental studies provided valuable insights into the boiling phenomena, however these studies provided often contradictory data due to internal recalculation (state of chaos in the fluid not applying to classical thermodynamic methods of calculation, therefore giving wrong return values) and have not provided conclusive findings yet to develop models and correlations.
[1] The nucleate boiling regime is important to engineers because of the high heat fluxes possible with moderate temperature differences.
where: The variable n depends on the surface fluid combination and typically has a value of 1.0 or 1.7.
If the heat flux of a boiling system is higher than the critical heat flux (CHF) of the system, the bulk fluid may boil, or in some cases, regions of the bulk fluid may boil where the fluid travels in small channels.
This results in a departure from nucleate boiling (DNB) in which steam bubbles no longer break away from the solid surface of the channel, bubbles dominate the channel or surface, and the heat flux dramatically decreases.
Vapor essentially insulates the bulk liquid from the hot surface.
Avoiding the CHF is an engineering problem in heat transfer applications, such as nuclear reactors, where fuel plates must not be allowed to overheat.
DNB may be avoided in practice by increasing the pressure of the fluid, increasing its flow rate, or by utilizing a lower temperature bulk fluid which has a higher CHF.
During transition boiling of water, the bubble formation is so rapid that a vapor film or blanket begins to form at the surface.
However, at any point on the surface, the conditions may oscillate between film and nucleate boiling, but the fraction of the total surface covered by the film increases with increasing temperature difference.