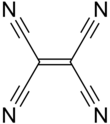
Tetracyanoethylene
TCNE is prepared by brominating malononitrile in the presence of potassium bromide to give the KBr-complex, and dehalogenating with copper.
[1] Oxidation of TCNE with hydrogen peroxide gives the corresponding epoxide, which has unusual properties.
Cyano groups have low energy π* orbitals, and the presence of four such groups, with their π systems (conjugated) to the central C=C double bond, gives rise to an electrophilic alkene.
[6] Upon reduction, this bond elongates to 141–145 pm, depending on the counterion.
[7] TCNE hydrolyzes in moist air to give hydrogen cyanide and should be handled accordingly.