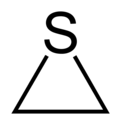
Thiirane
Thiirane, more commonly known as ethylene sulfide, is the cyclic chemical compound with the formula C2H4S.
Like many organosulfur compounds, this species has a highly unpleasant odour.
Thiirane is also used to describe any derivative of the parent ethylene sulfide.
According to electron diffraction, the C-C and C-S distances in ethylene sulfide are respectively 1.473 and 1.811 Å.
Ethylenesulfide adds to amines to afford 2-mercaptoethylamines,[6] which are good chelating ligands.