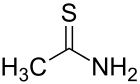
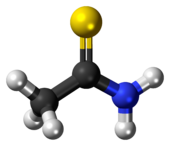
Thioacetamide
This white crystalline solid is soluble in water and serves as a source of sulfide ions in the synthesis of organic and inorganic compounds.
Thioacetamide is known to induce acute or chronic liver disease (fibrosis and cirrhosis) in the experimental animal model.
Its administration in rat induces hepatic encephalopathy, metabolic acidosis, increased levels of transaminases, abnormal coagulation, and centrilobular necrosis, which are the main features of the clinical chronic liver disease so thioacetamide can precisely replicate the initiation and progression of human liver disease in an experimental animal model.
[3] Thioacetamide is widely used in classical qualitative inorganic analysis as an in situ source for sulfide ions.
Thioacetamide is prepared by treating acetamide with phosphorus pentasulfide as shown in the following idealized reaction:[4] The C2NH2S portion of the molecule is planar; the C-S, C-N, and C-C distances are 1.68, 1.31, and 1.50 Å, respectively.