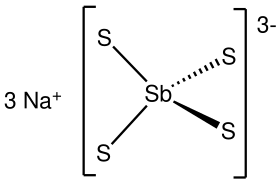
Sodium thioantimoniate
The nonahydrate consists of the tetrahedral tetrathioantimonate(V) anions SbS3−4 and sodium cations Na+, which are hydrated.
[1][2] Related salts are known for different cations including ammonium and potassium.
The anhydrous salt is a polymer with tetrahedral Na and Sb sites.
[3] Sodium tetrathioantimonate nonahydrate is prepared by the reaction of "antimony trisulfide", elemental sulfur, and aqueous sulfide source.
The required antimony trisulfide is prepared by treatment of Sb(III) compounds with sulfide sources: The hydrate dissolves in water to give the tetrahedral SbS3−4 ion.