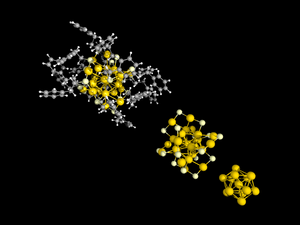
Thiolate-protected gold cluster
[3] If the synthesis is performed in a kinetically controlled manner, particularly stable representatives can be obtained with particles of uniform size (monodispersely), avoiding further separation steps.
The high affinity of the gold ions to electronegative and (partially) charged atoms of functional groups yields potential seeds for cluster formation.
Top-down synthesis of the clusters can be achieved by the "etching" of larger metallic nanoparticles with redox-active, thiol-containing biomolecules.
[7] The discrete optical transitions and occurrence of photoluminescence in these species are areas where they behave like molecular, rather than metallic, substances.
[8] According to this model they exhibit atomic-like electronic states, that are labeled S, P, D, F according to their respective angular momentum on the atomic level.
Such clusters can be synthesized monodispersely and are end products of the etching procedure after an addition of excess thiols does not lead to further metal dissolution.
[10] Worthy of note is that in 2013, a structural prediction of the Au130 (SCH3)50 cluster, based on Density Functional Theory (DFT) was confirmed in 2015.