Surface plasmon resonance
The simplest way to approach the problem is to treat each material as a homogeneous continuum, described by a frequency-dependent relative permittivity between the external medium and the surface.
This condition is met in the infrared-visible wavelength region for air/metal and water/metal interfaces (where the real dielectric constant of a metal is negative and that of air or water is positive).
LSPRs (localized surface plasmon resonances) are collective electron charge oscillations in metallic nanoparticles that are excited by light.
This field is highly localized at the nanoparticle and decays rapidly away from the nanoparticle/dielectric interface into the dielectric background, though far-field scattering by the particle is also enhanced by the resonance.
Light intensity enhancement is a very important aspect of LSPRs and localization means the LSPR has very high spatial resolution (subwavelength), limited only by the size of nanoparticles.
[4][5] In order to excite surface plasmon polaritons in a resonant manner, one can use electron bombardment or incident light beam (visible and infrared are typical).
S-polarized light (polarization occurs perpendicular to the plane of incidence) cannot excite electronic surface plasmons.
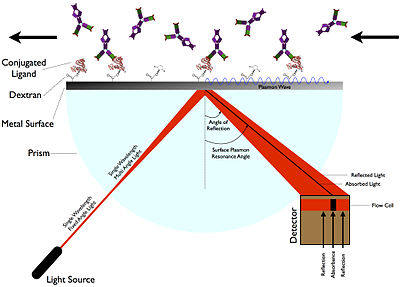
In the Otto configuration, the light illuminates the wall of a glass block, typically a prism, and is totally internally reflected.
SPR instruments consist of a light source, an input scheme, a prism with analyte interface, a detector, and computer.
The detectors used in surface plasmon resonance convert the photons of light reflected off the metallic film into an electrical signal.
This method provides a high contrast of the images based on the adsorbed amount of molecules, somewhat similar to Brewster angle microscopy (this latter is most commonly used together with a Langmuir–Blodgett trough).
[13] Shifts in this resonance due to changes in the local index of refraction upon adsorption to the nanoparticles can also be used to detect biopolymers such as DNA or proteins.
Related complementary techniques include plasmon waveguide resonance, QCM, extraordinary optical transmission, and dual-polarization interferometry.
[16][17][14] Additionally, the measurements on SPR can be followed real-time allowing the monitoring of individual steps in sequential binding events particularly useful in the assessment of for instance sandwich complexes.
Besides binding kinetics, MP-SPR can also provide information on structural changes in terms of layer true thickness and refractive index.
MP-SPR has been applied successfully in measurements of lipid targeting and rupture,[18] CVD-deposited single monolayer of graphene (3.7Å)[19] as well as micrometer thick polymers.
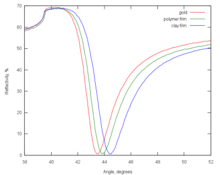
[20] The most common data interpretation is based on the Fresnel formulas, which treat the formed thin films as infinite, continuous dielectric layers.
In multi-parametric surface plasmon resonance, two SPR curves are acquired by scanning a range of angles at two different wavelengths, which results in a unique solution for both thickness and refractive index.
Due to the versatility of SPR instrumentation, this technique pairs well with other approaches, leading to novel applications in various fields, such as biomedical and environmental studies.
[21] In general, SPR biosensing is demonstrating advantages over other approaches in the biomedical field due to this technique being label-free, lower in costs, applicable in point-of-care settings, and capable of producing faster results for smaller research cohorts.
One of the first common applications of surface plasmon resonance spectroscopy was the measurement of the thickness (and refractive index) of adsorbed self-assembled nanofilms on gold substrates.
As SPR biosensors facilitate measurements at different temperatures, thermodynamic analysis can be performed to obtain a better understanding of the studied interaction.
As SPR allows real-time monitoring, individual steps in sequential binding events can be thoroughly assessed when investigating the suitability between antibodies in a sandwich configuration.
The large surface area of graphene also facilitates the immobilization of biomolecules while its low refractive index minimizes its interference.
For instance, the enhanced sensitivity of graphene can be used in conjunction with a silver SPR sensor, providing a cost-effective alternative for measuring glucose levels in urine.