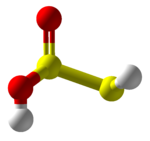
Thiosulfurous acid
Salts derived from thiosulfurous acid, which are also unknown, are named "thiosulfites", "thionosulfites" or "sulfurothioites".
Other possible isomers are dihydroxydisulfane or hypodithionous acid HOSSOH, a linear chain, and thiothionyl hydroxide (S=S(OH)2) a tautomer where the hydrogen has moved from a sulfur to an oxygen.
[7] It apparently decomposes to polysulfane oxide or polythionic acids in water, which is termed Wakenroder's liquid.
[5] In alkaline conditions thiosulfurous acid rapidly deteriorates forming a mixture of sulfide, sulfur, sulfite, and thiosulfate.
[9][10] The reaction with simple alkoxide sources with disulfur dichloride gives the unbranched ROSSOR.