Threonine protease
Threonine proteases are a family of proteolytic enzymes harbouring a threonine (Thr) residue within the active site.
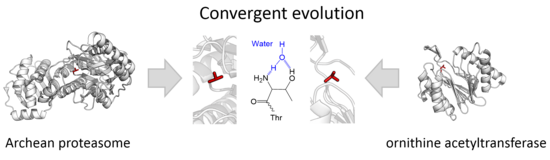
The prototype members of this class of enzymes are the catalytic subunits of the proteasome, however, the acyltransferases convergently evolved the same active site geometry and mechanism.
[1][2] The threonine must be N-terminal since the terminal amine of the same residue acts as a general base by polarising an ordered water which deprotonates the alcohol to increase its reactivity as a nucleophile.
[3][4] Catalysis takes place in two steps: Five families belonging to two separate superfamilies are currently recognised: the Ntn fold proteosomes[1] (superfamily PB) and the DOM fold ornithine acyltransferases[2] (superfamily PE).
The two superfamilies represent two independent, convergent evolutions of the same active site.