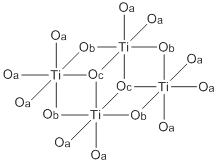
Titanium ethoxide
[3] Both Ti(OEt)4 exist mainly as tetramers with an octahedral coordination environment around the metal centers.
There are two types of titanium centers, depending on the number of terminal vs bridging alkoxide ligands.
[5] Like the ethoxide, titanium methoxide Ti(OMe)4 exists as a tetramer with each of the TiIV metal centers having an octahedral coordination environment.
Generally acid-catalysis yields a sol where the polymer chains are randomly oriented and linear.
In the base-mediated case bushy clusters or crosslinked networks are produced, these structures can trap solvent and reaction byproducts and form a gel coating.