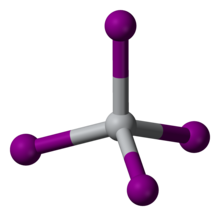
Titanium tetraiodide
TiI4 is a rare molecular binary metal iodide, consisting of isolated molecules of tetrahedral Ti(IV) centers.
[3] Reflecting its molecular character, TiI4 can be distilled without decomposition at one atmosphere; this property is the basis of its use in the van Arkel–de Boer process.
168 °C), reflecting the stronger intermolecular van der Waals bonding in the iodides.
[3] Three methods are well known: 1) From the elements, typically using a tube furnace at 425 °C:[4] This reaction can be reversed to produce highly pure films of Ti metal.
[6] TiI4 exhibits extensive reactivity toward alkenes and alkynes resulting in organoiodine derivatives.