Transition metal carboxylate complex
Carboxylates exhibit a variety of coordination modes, most common are κ1- (O-monodentate), κ2 (O,O-bidentate), and bridging.
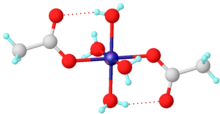
Carboxylates bind to single metals by one or both oxygen atoms, the respective notation being κ1- and κ2-.
One common example is hydrated nickel acetate, Ni(O2CCH3)2(H2O)4, which features intramolecular hydrogen-bonding between the uncoordinated oxygens and the protons of aquo ligands.
[6] Attempts to prepare some carboxylate complexes, especially for electrophilic metals, often gives oxo derivatives.
These clusters, called secondary bonding units (SBU's), are often linked by the conjugate bases of benzenedi- and tri- carboxylic acids.
[14] It has been claimed that "cobalt carboxylates are the most widely used homogeneous catalysts in industry" as they are used in the oxidation of p-xylene to terephthalic acid.
[15] Palladium(II) acetate has been described as being "among the most extensively used transition metal complexes in metal-mediated organic synthesis".
Many coupling reactions utilize this reagent, which is soluble in organic solvents and which contains a built-in Bronsted base (acetate).
[16] Dirhodium tetrakis(trifluoroacetate) is widely used catalyst for reactions involving diazo compounds.