Transition metal amino acid complexes
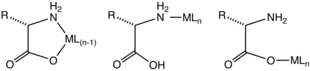
N,O bidentate amino carboxylates are "L-X" ligands in the Covalent bond classification method.
With respect to HSAB theory, N,O bidentate amino carboxylate is a pair of hard ligands.
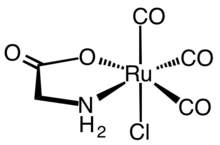
Using kinetically inert metal ions, complexes containing monodentate amino acids have been characterized.
Mixing simple metal salts with solutions of amino acids near neutral or elevated pH often affords bis- or tris complexes.
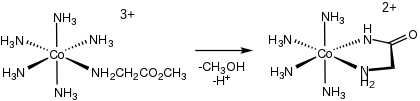
Both the violet meridional and red-pink facial isomers of tris(glycinato)cobalt(III) have been characterized[6] With L-alanine, L-leucine, and other amino acids, one obtains four stereoisomers.
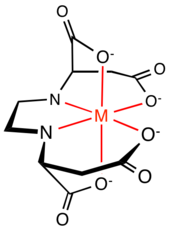
[8] Complexes with the 2:1 stoichiometry are illustrated by copper(II) glycinate [Cu(O2CC(R)HNH2)2], which exists both in anhydrous and pentacoordinate geometries.
In addition to the amino acids, peptides and proteins bind metal cofactors through their side chains.
The situation is more complicated for the N-terminal and O-terminal residues where the α-amino and carboxylate groups are unavailable, respectively.