Transition metal acyl complexes
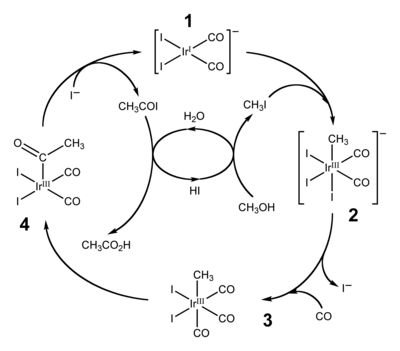
Such compounds occur as transient intermediates in many industrially useful reactions, especially carbonylations.
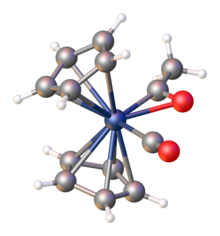
Monometallic acyl complexes adopt one of two related structures, C-bonded and η2-C-O-bonded.
Illustrative is the oxidative addition of acetyl chloride to Vaska's complex, converting square planar Ir(I) to octahedral Ir(III):[5][6] Some acyl complexes can be produced from aldehydes by C-H oxidative addition.
Coordinatively saturated metal carbonyls react with organolithium reagents to give acyls.
In practical sense, the most important reaction of metal acyls is their detachment by reductive elimination of aldehydes from acyl metal hydrides: This reaction is the final step of hydroformylation.