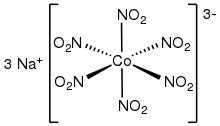
Transition metal nitrite complex
[2] Although the synthetic derivatives are only of scholarly interest, metal-nitrite complexes occur in several enzymes that participate in the nitrogen cycle.
The former two isomers have been characterized for the pentamminecobalt(III) system, i.e. [(NH3)5Co−NO2]2+ and [(NH3)5Co−ONO]2+, referred to as N-nitrito and O-nitrito, respectively.
In its reaction with ferric porphyrin complexes, nitrite gives the O-bonded isomer, Fe(porph)ONO.
Addition of donor ligands to this complex induces the conversion to the octahedral low-spin isomer, which now is a soft Lewis acid.
The potassium salts of [M(NO2)4]2− (M = Zn, Cd) feature homoleptic complexes with four O,O-bidentate nitrite ligands.
[12] Metal nitrito complexes figure prominently in the nitrogen cycle, which describes the relationships and interconversions of ammonia up to nitrate.
This protonation induces cleavage of an N–O bond, giving a HO–Cu–ON center, which features a nitric oxide ligand O-bonded to Cu(II) (an isonitrosyl).