Transition metal carbonate and bicarbonate complexes
The inventory of complexes is large, enhanced by the fact that the carbonate ligand can bind metal ions in a variety of bonding modes.
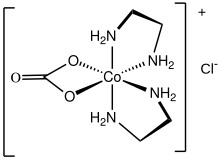
To a single metal ion, carbonate is observed to bind in both unidentate (κ1-) and bidentate (κ2-) fashions.
With two metals, the number of bonding modes increases because carbonate often serves as a bridging ligand.
In some cases, bicarbonate intermediates are implicated since carbonate does not exist in appreciable concentrations near neutral pH.
For example, the deoxygenation of peroxycarbonate by tertiary phosphines: Carbon dioxide undergoes disproportionation upon reaction with low-valence metals.
Protonation of bicarbonate ligands results in loss of carbon dioxide and formation of the metal hydroxide.
[10] In the biological sphere, zinc bicarbonate complexes are central intermediates in the action of the carbonic anhydrase:[11]