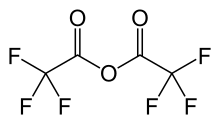
Trifluoroacetic anhydride
[2] The dehydration might also be carried out with excess α-halogenated acid chlorides.
For example, with dichloroacetyl chloride:[3] Trifluoroacetic anhydride has various uses in organic synthesis.
Other electrophilic aromatic substitution reactions can also be promoted with trifluoroacetic anhydride, including nitration, sulfonation and nitrosylation.
[4] It can be used in place of oxalyl chloride in the Swern oxidation, allowing temperatures up to −30 °C.
[5] With sodium iodide, it reduces sulfoxides to sulfides.