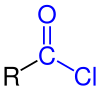
Acyl chloride
When other functional groups take priority, acyl chlorides are considered prefixes — chlorocarbonyl-:[1] Lacking the ability to form hydrogen bonds, acyl chlorides have lower boiling and melting points than similar carboxylic acids.
This structure undergoes an acyl substitution with the liberated chloride, forming the acid anhydride and releasing regenerated molecule of DMF.
The tetrahedral intermediate collapses, ejecting chloride ion as the leaving group and forming oxonium species 3.
The alcoholysis of acyl halides (the alkoxy-dehalogenation) is believed to proceed via an SN2 mechanism (Scheme 10).
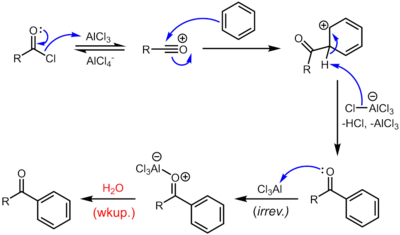
The use of two phases (aqueous for amine, organic for acyl chloride) is called the Schotten-Baumann reaction.
[24] Acid halides react with carbon nucleophiles, such as Grignards and enolates, although mixtures of products can result.
For example, when benzoyl chloride (1) is treated with two equivalents of a Grignard reagent, such as methyl magnesium bromide (MeMgBr), 2-phenyl-2-propanol (3) is obtained in excellent yield.
Although acetophenone (2) is an intermediate in this reaction, it is impossible to isolate because it reacts with a second equivalent of MeMgBr rapidly after being formed.
Unlike most other carbon nucleophiles, lithium dialkylcuprates – often called Gilman reagents – can add to acid halides just once to give ketones.
[27] Carbon nucleophiles such as Grignard reagents, convert acyl chlorides to ketones, which in turn are susceptible to the attack by second equivalent to yield the tertiary alcohol.
[28] The reaction with Gilman reagents also afford ketones, reflecting the low nucleophilicity of these lithium diorganocopper compounds.
[30][12][14] Because of the harsh conditions and the reactivity of the intermediates, this otherwise quite useful reaction tends to be messy, as well as environmentally unfriendly.
Illustrative is the oxidative addition of acetyl chloride to Vaska's complex, converting square planar Ir(I) to octahedral Ir(III):[31] Low molecular weight acyl chlorides are often lachrymators, and they react violently with water, alcohols, and amines.