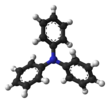
Triphenylamine
At room temperature it appears as a colorless crystalline solid, with monoclinic structure.
It is well miscible in diethyl ether and benzene, but it is practically insoluble in water, and partially in ethanol.
This arrangement prevents nitrogen protonation, a key mechanism for providing basicity to a solution.
From this characteristic, moreover, it follows that the three N-C bonds all lie on the same plane and that they are located at 120° from each other, which is not the case with aliphatic amines and ammonia, where the orbitals of nitrogen are arranged in a tetrahedron.
Due to steric hindrance, the phenyl groups are not on the same plane defined by the three N-C bonds, but are twisted, giving the molecule its characteristic "propeller-like" shape.