Cancer immunotherapy
Surgical wounds were left open to facilitate the development of infection, and purulent sores were created deliberately... One of the most well-known effects of microorganisms on ... cancer was reported in 1891, when an American surgeon, William Coley, inoculated patients having inoperable tumours with [ Streptococcus pyogenes ].
"[4] "Coley [had] thoroughly reviewed the literature available at that time and found 38 reports of cancer patients with accidental or iatrogenic feverish erysipelas.
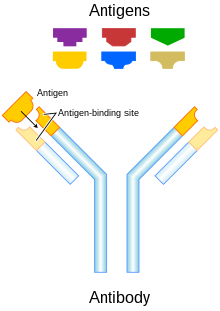
Passive antibody therapies aim to increase the activity of the immune system without specifically targeting cancer cells.
Other adjuvants include proteins or other chemicals that attract and/or activate dendritic cells, such as granulocyte-macrophage colony-stimulating factor (GM-CSF).
[citation needed] Sipuleucel-T (Provenge) was approved for treatment of asymptomatic or minimally symptomatic metastatic castration-resistant prostate cancer in 2010.
The treatment consists of removal of antigen-presenting cells from blood by leukapheresis and growing them with the fusion protein PA2024 made from GM-CSF and prostate-specific prostatic acid phosphatase (PAP) and reinfused.
[25] The simplest example involves removing TILs from a tumor, culturing but not modifying them, and infusing the result back into the tumour.
Tisagenlecleucel (Kymriah), a chimeric antigen receptor (CAR-T) therapy, was approved by the FDA in 2017 to treat acute lymphoblastic leukemia (ALL).
Axicabtagene ciloleucel (Yescarta) is another CAR-T therapeutic, approved in 2017 for treatment of diffuse large B-cell lymphoma (DLBCL).
And because the cells are younger, they last longer in the body, show stronger potency against cancer, and display fewer markers of exhaustion.
TCR-T therapies use heterodimers made of alpha and beta peptide chains to recognize MHC-presented polypeptide fragment molecules.
Fc regions are varied: they exist in numerous subtypes and can be further modified, for example with the addition of sugars in a process called glycosylation.
Due to the antibody target (cells of the immune system), common complications of alemtuzumab therapy are infection, toxicity and myelosuppression.
[76] The most common side effects when used on its own include tiredness, reduced appetite, nausea, vomiting, cough, difficulty breathing, diarrhea, rash, fever, pain in the back, joints, muscles and bones, weakness, itching and urinary tract infection.
[84] Common side effects include fatigue, musculoskeletal pain, diarrhea, nausea, infusion-related reactions, rash, decreased appetite and swelling of the limbs (peripheral edema).
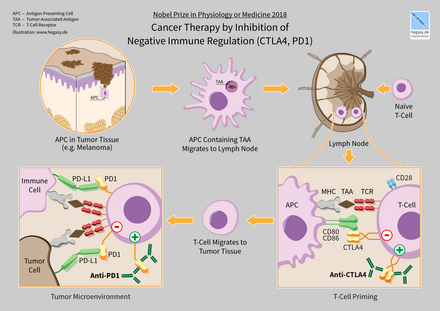
[85] Durvalumab (Imfinzi) is a human immunoglobulin G1 kappa (IgG1κ) monoclonal antibody that blocks the interaction of programmed cell death ligand 1 (PD-L1) with the PD-1 and CD80 (B7.1) molecules.
Durvalumab is approved for the treatment of patients with locally advanced or metastatic urothelial carcinoma who: On 16 February 2018, the Food and Drug Administration approved durvalumab for patients with unresectable stage III non-small cell lung cancer (NSCLC) whose disease has not progressed following concurrent platinum-based chemotherapy and radiation therapy.
[89] Elotuzumab, sold under the brand name Empliciti, is a humanized IgG1 monoclonal antibody medication used in combination with lenalidomide and dexamethasone, for adults that have received 1 to 3 prior therapies for the treatment of multiple myeloma.
[92] It is also indicated for adult patients in combination with pomalidomide and dexamethasone, who have received 2 prior therapies including lenalidomide and a protease inhibitor.
[94] Common side effects of elotuzumab with lenalidomide and dexamethasone includes fatigue, diarrhea, pyrexia, constipation, cough, peripheral neuropathy, nasopharyngitis, upper respiratory tract infection, decreased appetite, and pneumonia.
A plethora of possible reasons for the absence of efficacy in many patients have been proposed, but the biomedical community has still to begin to find consensus in this respect.
For instance, a recent paper documented that infection with Helicobacter pylori would negatively influence the effects of immunocheckpoint inhibitors in gastric cancer.,[151] but this notion was quickly challenged by others.
"Ipilimumab in combination with paclitaxel and carboplatin as first-line treatment in stage IIIB/IV non-small-cell lung cancer: results from a randomized, double-blind, multicenter phase II study".
[159] At the same time the levels of circulating antibodies were lower, suggesting that local administration of the anti-CTLA-4 therapy might result in fewer adverse events.
[160] A 2016 clinical trial for non-small cell lung cancer failed to meet its primary endpoint for treatment in the first-line setting, but is FDA-approved in subsequent lines of therapy.
For example, the FDA approved BRAF-associated medication for metastatic melanoma, to be administered to patients after testing for the BRAF genetic mutation.
Oncolytic viruses are thought not only to cause direct destruction of the tumour cells, but also to stimulate host anti-tumour immune responses for long-term immunotherapy.
The presence of CD8+ T cells in cancer lesions, as identified using RNA sequencing data, is higher in tumors with a high mutational burden.
In non–small cell lung cancer patients treated with lambrolizumab, mutational load shows a strong correlation with clinical response.
[202] In the 1980s, Japan's Ministry of Health, Labour and Welfare approved polysaccharide-K extracted from the mushroom, Coriolus versicolor, to stimulate the immune systems of patients undergoing chemotherapy.