Fermium
Fermium was discovered in the debris of the first hydrogen bomb explosion in 1952, and named after Enrico Fermi, one of the pioneers of nuclear physics.
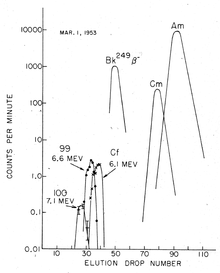
[6][7][8] Initial examination of the debris from the explosion had shown the production of a new isotope of plutonium, 24494Pu: this could only have formed by the absorption of six neutrons by a uranium-238 nucleus followed by two β− decays.
[8] The discovery of the new elements, and the new data on neutron capture, was initially kept secret on the orders of the U.S. military until 1955 due to Cold War tensions.
[9] The Berkeley team had been worried that another group might discover lighter isotopes of element 100 through ion-bombardment techniques before they could publish their classified research,[8] and this proved to be the case.
A group at the Nobel Institute for Physics in Stockholm independently discovered the element, producing an isotope later confirmed to be 250Fm (t1/2 = 30 min) by bombarding a 23892U target with oxygen-16 ions, and published their work in May 1954.
[13] Nevertheless, the priority of the Berkeley team was generally recognized, and with it the prerogative to name the new element in honour of Enrico Fermi, the developer of the first artificial self-sustained nuclear reactor.
[c][20] The major source is the 85 MW High Flux Isotope Reactor (HFIR) at the Oak Ridge National Laboratory in Tennessee, USA, which is dedicated to the production of transcurium (Z > 96) elements.
This is usually achieved by ion-exchange chromatography, with the standard process using a cation exchanger such as Dowex 50 or TEVA eluted with a solution of ammonium α-hydroxyisobutyrate.
Whereas it was hoped to discover new chemical elements heavier than fermium, those were not found after a series of megaton explosions conducted between 1954 and 1956 at the atoll.
They were less successful in terms of yield, which was attributed to stronger losses of heavy isotopes due to enhanced fission rates in heavy-element charges.
In the dependence on the atomic mass number, the yield showed a saw-tooth behavior with the lower values for odd isotopes, due to their higher fission rates.
Aircraft filters adsorbed only about 4×10−14 of the total amount and collection of tons of corals at Enewetak Atoll increased this fraction by only two orders of magnitude.
This observation demonstrated the highly nonlinear dependence of the transuranium elements yield on the amount of retrieved radioactive rock.
Synthesis of fermium from naturally occurring uranium and thorium in the Earth's crust requires multiple neutron captures, which is extremely unlikely.
Under normal conditions, fermium exists in solution as the Fm3+ ion, which has a hydration number of 16.9 and an acid dissociation constant of 1.6×10−4 (pKa = 3.8).
[44] Though few people come in contact with fermium, the International Commission on Radiological Protection has set annual exposure limits for the two most stable isotopes.