Uranium
It occurs naturally in low concentrations of a few parts per million in soil, rock and water, and is commercially extracted from uranium-bearing minerals such as uraninite.
[10] The 1789 discovery of uranium in the mineral pitchblende is credited to Martin Heinrich Klaproth, who named the new element after the recently discovered planet Uranus.
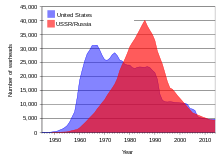
An ensuing arms race during the Cold War between the United States and the Soviet Union produced tens of thousands of nuclear weapons that used uranium metal and uranium-derived plutonium-239.
[12] Depleted uranium is preferred over similarly dense metals due to its ability to be easily machined and cast as well as its relatively low cost.
Later, a much more complicated and far more powerful type of fission/fusion bomb (thermonuclear weapon) was built, that uses a plutonium-based device to cause a mixture of tritium and deuterium to undergo nuclear fusion.
Such bombs are jacketed in a non-fissile (unenriched) uranium case, and they derive more than half their power from the fission of this material by fast neutrons from the nuclear fusion process.
[12] The use of pitchblende, uranium in its natural oxide form, dates back to at least the year 79 AD, when it was used in the Roman Empire to add a yellow color to ceramic glazes.
[12] Yellow glass with 1% uranium oxide was found in a Roman villa on Cape Posillipo in the Gulf of Naples, Italy, by R. T. Gunther of the University of Oxford in 1912.
[29] Starting in the late Middle Ages, pitchblende was extracted from the Habsburg silver mines in Joachimsthal, Bohemia (now Jáchymov in the Czech Republic) in the Ore Mountains, and was used as a coloring agent in the local glassmaking industry.
Tools made with these formulas remained in use for several decades,[35][36] until the Manhattan Project and the Cold War placed a large demand on uranium for fission research and weapon development.
[37] The fission products were at first mistaken for new elements with atomic numbers 93 and 94, which the Dean of the Sapienza University of Rome, Orso Mario Corbino, named ausenium and hesperium, respectively.
Lise Meitner and her nephew, physicist Otto Robert Frisch, published the physical explanation in February 1939 and named the process "nuclear fission".
Despite fission having been discovered in Germany, the Uranverein ("uranium club") Germany's wartime project to research nuclear power and/or weapons was hampered by limited resources, infighting, the exile or non-involvement of several prominent scientists in the field and several crucial mistakes such as failing to account for impurities in available graphite samples which made it appear less suitable as a neutron moderator than it is in reality.
[44] On 2 December 1942, as part of the Manhattan Project, another team led by Enrico Fermi was able to initiate the first artificial self-sustained nuclear chain reaction, Chicago Pile-1.
[49][50] The world's first commercial scale nuclear power station, Obninsk in the Soviet Union, began generation with its reactor AM-1 on 27 June 1954.
[53] Above-ground nuclear tests by the Soviet Union and the United States in the 1950s and early 1960s and by France into the 1970s and 1980s[22] spread a significant amount of fallout from uranium daughter isotopes around the world.
[56] The Radiation Exposure Compensation Act, a 1990 law in the US, required $100,000 in "compassion payments" to uranium miners diagnosed with cancer or other respiratory ailments.
[18] Police in Asia, Europe, and South America on at least 16 occasions from 1993 to 2005 have intercepted shipments of smuggled bomb-grade uranium or plutonium, most of which was from ex-Soviet sources.
[18] From 1993 to 2005 the Material Protection, Control, and Accounting Program, operated by the federal government of the United States, spent about US$550 million to help safeguard uranium and plutonium stockpiles in Russia.
The number of employees receiving annual radiation doses above 20 mSv, which is equivalent to a single full-body CT scan,[58] saw a strong decline around 2000.
In November 2015, the Russian government approved a federal program for nuclear and radiation safety for 2016 to 2030 with a budget of 562 billion rubles (ca.
[65][66] Besides the two extant primordial uranium isotopes, 235U and 238U, the r-process also produced significant quantities of 236U, which has a shorter half-life and so is an extinct radionuclide, having long since decayed completely to 232Th.
[71][72] Other organisms, such as the lichen Trapelia involuta or microorganisms such as the bacterium Citrobacter, can absorb concentrations of uranium that are up to 300 times the level of their environment.
Extensive measures must be employed to extract the metal from its ore.[79] High-grade ores found in Athabasca Basin deposits in Saskatchewan, Canada can contain up to 23% uranium oxides on average.
[92][93] In 2005, ten countries accounted for the majority of the world's concentrated uranium oxides: Canada (27.9%), Australia (22.8%), Kazakhstan (10.5%), Russia (8.0%), Namibia (7.5%), Niger (7.4%), Uzbekistan (5.5%), the United States (2.5%), Argentina (2.1%) and Ukraine (1.9%).
In 2021, its share was 45.1%, followed by Namibia (11.9%), Canada (9.7%), Australia (8.7%), Uzbekistan (7.2%), Niger (4.7%), Russia (5.5%), China (3.9%), India (1.3%), Ukraine (0.9%), and South Africa (0.8%), with a world total production of 48,332 tonnes.
[109] Nevertheless, the two most stable isotopes, 238U and 235U, have half-lives long enough to occur in nature as primordial radionuclides, with measurable quantities having survived since the formation of the Earth.
Owing to the fissility of 233U and the greater natural abundance of thorium (three times that of uranium),[116] 233U has been investigated for use as nuclear fuel as a possible alternative to 235U and 239Pu,[117] though is not in widespread use as of 2022[update].
[11] The only significant deviation from the 235U to 238U ratio in any known natural samples occurs in Oklo, Gabon, where natural nuclear fission reactors consumed some of the 235U some two billion years ago when the ratio of 235U to 238U was more akin to that of low enriched uranium allowing regular ("light") water to act as a neutron moderator akin to the process in humanmade light water reactors.
The existence of such natural fission reactors which had been theoretically predicted beforehand was proven as the slight deviation of 235U concentration from the expected values were discovered during uranium enrichment in France.