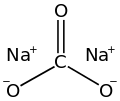
Sodium carbonate
Alkalinity affects gluten production in kneaded doughs, and also improves browning by reducing the temperature at which the Maillard reaction occurs.
To take advantage of the former effect, sodium carbonate is therefore one of the components of kansui (かん水), a solution of alkaline salts used to give Japanese ramen noodles their characteristic flavour and chewy texture; a similar solution is used in Chinese cuisine to make lamian, for similar reasons.
Cantonese bakers similarly use sodium carbonate as a substitute for lye-water to give moon cakes their characteristic texture and improve browning.
While it is less likely to cause chemical burns than lye, care must still be taken when working with sodium carbonate in the kitchen, as it is corrosive to aluminum cookware, utensils, and foil.
As a common alkali, it is preferred in many chemical processes because it is cheaper than sodium hydroxide and far safer to handle.
For example, it is used as a pH regulator to maintain stable alkaline conditions necessary for the action of the majority of photographic film developing agents.
It is also a common additive in swimming pools and aquarium water to maintain a desired pH and carbonate hardness (KH).
It is also used in the froth flotation process to maintain a favourable pH as a float conditioner besides CaO and other mildly basic compounds.
Although NaHCO3 is itself an intermediate product of the Solvay process, the heating needed to remove the ammonia that contaminates it decomposes some NaHCO3, making it more economical to react finished Na2CO3 with CO2: In a related reaction, sodium carbonate is used to make sodium bisulfite (NaHSO3), which is used for the "sulfite" method of separating lignin from cellulose.
Sodium carbonate is used by the brick industry as a wetting agent to reduce the amount of water needed to extrude the clay.
Sodium carbonate is used in toothpastes, where it acts as a foaming agent and an abrasive, and to temporarily increase mouth pH.
[6] Sodium carbonate is soluble in water, and can occur naturally in arid regions, especially in mineral deposits (evaporites) formed when seasonal lakes evaporate.
Deposits have been identified as the source of bright spots on Ceres, interior material that has been brought to the surface.
Large natural deposits found in 1938, such as the one near Green River, Wyoming, have made mining more economical than industrial production in North America.
"Barilla" is a commercial term applied to an impure form of potash obtained from coastal plants or kelp.
Plant and seaweed sources for soda ash, and also for the related alkali "potash", became increasingly inadequate by the end of the 18th century, and the search for commercially viable routes to synthesizing soda ash from salt and other chemicals intensified.
[27] In 1792, the French chemist Nicolas Leblanc patented a process for producing sodium carbonate from salt, sulfuric acid, limestone, and coal.
The hydrochloric acid produced by the Leblanc process was a major source of air pollution, and the calcium sulfide byproduct also presented waste disposal issues.